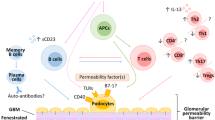
Abstract
Idiopathic nephrotic syndrome (INS) is a common glomerular disease in childhood, and the immunological involvement in the pathogenesis of non-genetic INS, although not fully elucidated, is evident. This narrative review aims to offer a concise and in-depth view of the current knowledge on the immunological mechanisms of the development of INS as well as the role of the immunological components of the disease in the responsiveness to treatment. T cell immunity appears to play a major role in the INS immunopathogenesis and has been the first to be linked to the disease. Various T cell immunophenotypes are implicated in INS, including T-helper-1, T-helper-2, T-helper-17, and T regulatory cells, and various cytokines have been proposed as surrogate biomarkers of the disease; however, no distinct T helper or cytokine profile has been conclusively linked to the disease. More recently, the recognition of the role of B cell mediated immunity and the various B cell subsets that are dysregulated in patients with INS have led to new hypotheses on the underlying immunological causes of INS. Finally, the disambiguation of the exact mechanisms of the INS development in the future may be the key to the development of more targeted personalized approaches in managing INS.
Conclusions: INS demonstrates particularly interesting immunopathogenetic pathways, in which multiple interactions between T cell and B cell immunity and the podocyte are involved. The disambiguation of these pathways will provide promising novel therapeutic targets in INS.
What is Known: • INS is the most common glomerular disease in the paediatric population, and its onset and relapses have been linked to various immunological triggers. • Multiple immunological mechanisms have been implicated in the pathogenesis of INS; however, no single distinct immunological profile has been recognized. | |
What is New: • Th17 cells and Treg cells play an important role in the immune dysregulation in INS. • Transitional B cell levels as well as the transitional/memory B cell ratio have been correlated to nephrotic relapses and have been proposed as biomarkers of INS relapses in SSNS patients. |


Similar content being viewed by others
Availability of data and material
Not applicable.
Code availability
Not applicable.
Abbreviations
- CNIs:
-
Calcineurin inhibitors
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- EBNA1:
-
Epstein-Barr nuclear antigen 1
- FSGS:
-
Focal segmental glomerulosclerosis
- HLA:
-
Human leukocyte antigen
- INS:
-
Idiopathic nephrotic syndrome
- IL:
-
Interleukin
- IL-2R:
-
IL-2 receptor
- IL-4Rα:
-
IL-4 receptor alpha chain
- MHC:
-
Major histocompatibility complex
- MCD:
-
Minimal change disease
- SRNS:
-
Steroid-resistant nephrotic syndrome
- SSNS:
-
Steroid-sensitive nephrotic syndrome
- STAT6:
-
Signal transducer and activator of transcription 6
- Th1:
-
T-helper 1
- Th17:
-
T-helper 17
- Th2:
-
T-helper 2
- Treg:
-
T-regulatory
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumour necrosis factor-alpha
- UCHL1:
-
Ubiquitin carboxyl-terminal hydrolase L1
- ZO-1:
-
Zonula occludens-1
References
Noone DG, Iijima K, Parekh R (2018) Idiopathic nephrotic syndrome in children. Lancet 392:61–74. https://doi.org/10.1016/S0140-6736(18)30536-1
Müller-Deile J, Schiffer M (2016) Podocyte directed therapy of nephrotic syndrome—can we bring the inside out? Pediatr Nephrol 31:393–405. https://doi.org/10.1007/s00467-015-3116-4
Eddy AA, Symons JM (2003) Nephrotic syndrome in childhood. Lancet (London, England) 362:629–639. https://doi.org/10.1016/S0140-6736(03)14184-0
Dossier C, Lapidus N, Bayer F et al (2016) Epidemiology of idiopathic nephrotic syndrome in children: endemic or epidemic? Pediatr Nephrol 31:2299–2308. https://doi.org/10.1007/s00467-016-3509-z
Shalhoub RJ (1974) Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet (London, England) 2:556–560. https://doi.org/10.1016/s0140-6736(74)91880-7
Gupta A, Quigg RJ (2015) Glomerular diseases associated with hepatitis B and C. Adv Chronic Kidney Dis 22:343–351. https://doi.org/10.1053/j.ackd.2015.06.003
Dossier C, Sellier-Leclerc A-L, Rousseau A et al (2014) Prevalence of herpesviruses at onset of idiopathic nephrotic syndrome. Pediatr Nephrol 29:2325–2331. https://doi.org/10.1007/s00467-014-2860-1
Wei C-C, Tsai J-D, Lin C-L et al (2014) Increased risk of idiopathic nephrotic syndrome in children with atopic dermatitis. Pediatr Nephrol 29:2157–2163. https://doi.org/10.1007/s00467-014-2835-2
Wei C-C, Lin C-L, Shen T-C, Sung F-C (2015) Occurrence of common allergic diseases in children with idiopathic nephrotic syndrome. J Epidemiol 25:370. https://doi.org/10.2188/JEA.JE20140167
Debiec H, Dossier C, Letouzé E et al (2018) Transethnic, genome-wide analysis reveals immune-related risk alleles and phenotypic correlates in pediatric steroid-sensitive nephrotic syndrome. J Am Soc Nephrol 29:2000–2013. https://doi.org/10.1681/ASN.2017111185
Baris HE, Baris S, Karakoc-Aydiner E et al (2016) The effect of systemic corticosteroids on the innate and adaptive immune system in children with steroid responsive nephrotic syndrome. Eur J Pediatr 175:685–693. https://doi.org/10.1007/s00431-016-2694-x
Bhatia D, Sinha A, Hari P et al (2018) Rituximab modulates T- and B-lymphocyte subsets and urinary CD80 excretion in patients with steroid-dependent nephrotic syndrome. Pediatr Res 84:520–526. https://doi.org/10.1038/s41390-018-0088-7
Liu L, Qin Y, Cai J et al (2011) Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol 139:314–320. https://doi.org/10.1016/j.clim.2011.02.018
Chung CF, Kitzler T, Kachurina N et al (2019) Intrinsic tumor necrosis factor-α pathway is activated in a subset of patients with focal segmental glomerulosclerosis. PLoS ONE 14. https://doi.org/10.1371/journal.pone.0216426
Otalora L, Chavez E, Watford D et al (2019) Identification of glomerular and podocyte-specific genes and pathways activated by sera of patients with focal segmental glomerulosclerosis. PLoS ONE 14. https://doi.org/10.1371/journal.pone.0222948
Youssef DM, El-Shal AS, Hussein S et al (2018) Tumor necrosis factor alpha gene polymorphisms and haplotypes in Egyptian children with nephrotic syndrome. Cytokine 102:76–82. https://doi.org/10.1016/j.cyto.2017.06.021
Weissbach A, Garty BZ, Lagovsky I et al (2017) Serum tumor necrosis factor-alpha levels in children with nephrotic syndrome: a pilot study. Isr Med Assoc J 19:30–33
Zea AH, Stewart T, Ascani J et al (2016) Activation of the IL-2 receptor in podocytes: a potential mechanism for podocyte injury in idiopathic nephrotic syndrome? PLoS ONE 11:e0157907. https://doi.org/10.1371/journal.pone.0157907
Shimoyama H, Nakajima M, Naka H et al (2004) Up-regulation of interleukin-2 mRNA in children with idiopathic nephrotic syndrome. Pediatr Nephrol 19:1115–1121. https://doi.org/10.1007/s00467-004-1569-y
Kalavrizioti D, Gerolymos M, Rodi M et al (2015) T helper (Th)-cytokines in the urine of patients with primary glomerulonephritis treated with immunosuppressive drugs: Can they predict outcome? Cytokine 76:260–269. https://doi.org/10.1016/j.cyto.2015.08.002
Printza N, Papachristou F, Tzimouli V et al (2008) IL-18 is correlated with type-2 immune response in children with steroid sensitive nephrotic syndrome. Cytokine 44:262–268. https://doi.org/10.1016/j.cyto.2008.08.012
Stangou M, Spartalis Μ, Daikidou D-V et al (2016) Impact of Τh1 and Τh2 cytokines in the progression of idiopathic nephrotic syndrome due to focal segmental glomerulosclerosis and minimal change disease. J Nephropathol 6:187–195. https://doi.org/10.15171/jnp.2017.32
Zhou J, Shi F, Xun W (2018) Leptin, hs-CRP, IL-18 and urinary protein before and after treatment of children with nephrotic syndrome. Exp Ther Med 15:4426–4430. https://doi.org/10.3892/etm.2018.5923
Kim AH, Chung J-J, Akilesh S et al (2017) B cell-derived IL-4 acts on podocytes to induce proteinuria and foot process effacement. JCI insight 2. https://doi.org/10.1172/jci.insight.81836
Ikeuchi Y, Kobayashi Y, Arakawa H et al (2009) Polymorphisms in interleukin-4-related genes in patients with minimal change nephrotic syndrome. Pediatr Nephrol 24:489–495. https://doi.org/10.1007/s00467-008-1003-y
Ha T-S, Nam JA, Seong S-B et al (2017) Montelukast improves the changes of cytoskeletal and adaptor proteins of human podocytes by interleukin-13. Inflamm Res 66:793–802. https://doi.org/10.1007/s00011-017-1058-y
Park SJ, Saleem MA, Nam J-A et al (2015) Effects of interleukin-13 and montelukast on the expression of zonula occludens-1 in human podocytes. Yonsei Med J 56:426–432. https://doi.org/10.3349/ymj.2015.56.2.426
Tsuji S, Akagawa S, Akagawa Y et al (2021) Idiopathic nephrotic syndrome in children: role of regulatory T cells and gut microbiota. Pediatr Res 89:1185–1191. https://doi.org/10.1038/s41390-020-1022-3
Guimarães FTL, Ferreira RN, Brito-Melo GEA et al (2019) Pediatric patients with steroid-sensitive nephrotic syndrome have higher expression of T regulatory lymphocytes in comparison to steroid-resistant disease. Front Pediatr 7:114. https://doi.org/10.3389/fped.2019.00114
Chan C-Y, Teo S, Lu L et al (2021) Low regulatory T-cells: a distinct immunological subgroup in minimal change nephrotic syndrome with early relapse following rituximab therapy. Transl Res. https://doi.org/10.1016/j.trsl.2021.03.019
Wang L, Li Q, Wang L et al (2013) The role of Th17/IL-17 in the pathogenesis of primary nephrotic syndrome in children. Kidney Blood Press Res 37:332–345. https://doi.org/10.1159/000350161
Shao XS, Yang XQ, Zhao XD et al (2009) The prevalence of Th17 cells and FOXP3 regulate T cells (Treg) in children with primary nephrotic syndrome. Pediatr Nephrol 24:1683–1690. https://doi.org/10.1007/s00467-009-1194-x
Al-Eisa A, Al Rushood M, Al-Attiyah R (2017) Urinary excretion of IL-1β, IL-6 and IL-8 cytokines during relapse and remission of idiopathic nephrotic syndrome. J Inflamm Res 10:1–5. https://doi.org/10.2147/JIR.S124947
May CJ, Welsh GI, Chesor M et al (2019) Human Th17 cells produce a soluble mediator that increases podocyte motility via signaling pathways that mimic PAR-1 activation. Am J Physiol Renal Physiol 317:F913–F921. https://doi.org/10.1152/ajprenal.00093.2019
Goldwich A, Burkard M, Olke M et al (2013) Podocytes are nonhematopoietic professional antigen-presenting cells. J Am Soc Nephrol 24:906–916. https://doi.org/10.1681/ASN.2012020133
Reiser J, von Gersdorff G, Loos M et al (2004) Induction of B7–1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113:1390–1397. https://doi.org/10.1172/JCI20402
Garin EH, Diaz LN, Mu W et al (2009) Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol 20:260–266. https://doi.org/10.1681/ASN.2007080836
Ishimoto T, Cara-Fuentes G, Wang H et al (2013) Serum from minimal change patients in relapse increases CD80 expression in cultured podocytes. Pediatr Nephrol 28:1803–1812. https://doi.org/10.1007/s00467-013-2498-4
Garin EH, Mu W, Arthur JM et al (2010) Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int 78:296–302. https://doi.org/10.1038/ki.2010.143
Ling C, Liu X, Shen Y et al (2015) Urinary CD80 levels as a diagnostic biomarker of minimal change disease. Pediatr Nephrol 30:309–316. https://doi.org/10.1007/s00467-014-2915-3
Ling C, Liu X, Shen Y et al (2018) Urinary CD80 excretion is a predictor of good outcome in children with primary nephrotic syndrome. Pediatr Nephrol 33:1183–1187. https://doi.org/10.1007/s00467-018-3885-7
Eroglu FK, Orhan D, İnözü M et al (2019) CD80 expression and infiltrating regulatory T cells in idiopathic nephrotic syndrome of childhood. Pediatr Int 61:1250–1256. https://doi.org/10.1111/ped.14005
Sellier-Leclerc A-L, Duval A, Riveron S et al (2007) A humanized mouse model of idiopathic nephrotic syndrome suggests a pathogenic role for immature cells. J Am Soc Nephrol 18:2732–2739. https://doi.org/10.1681/ASN.2006121346
Lapillonne H, Leclerc A, Ulinski T et al (2008) Stem cell mobilization in idiopathic steroid-sensitive nephrotic syndrome. Pediatr Nephrol 23:1251–1256. https://doi.org/10.1007/s00467-008-0793-2
Ahmed MS, Wong CF (2007) Rituximab and nephrotic syndrome: a new therapeutic hope? Nephrol Dial Transplant 23:11–17. https://doi.org/10.1093/ndt/gfm683
Kallash M, Smoyer WE, Mahan JD (2019) Rituximab use in the management of childhood nephrotic syndrome. Front Pediatr 7:178. https://doi.org/10.3389/fped.2019.00178
Printza N, Papachristou F, Tzimouli V et al (2009) Peripheral CD19+ B cells are increased in children with active steroid-sensitive nephrotic syndrome. NDT Plus 2:435–436. https://doi.org/10.1093/ndtplus/sfp087
Ravani P, Ponticelli A, Siciliano C et al (2013) Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 84:1025–1033. https://doi.org/10.1038/ki.2013.211
Ling C, Wang X, Chen Z et al (2019) Altered B-lymphocyte homeostasis in idiopathic nephrotic syndrome. Front Pediatr 7. https://doi.org/10.3389/fped.2019.00377
Ling C, Chen Z, Fan J et al (2020) Decreased circulating transitional B-cell to memory B-cell ratio is a risk factor for relapse in children with steroid-sensitive nephrotic syndrome. Nephron. https://doi.org/10.1159/000511319
Colucci M, Carsetti R, Cascioli S et al (2019) B cell phenotype in pediatric idiopathic nephrotic syndrome. Pediatr Nephrol 34:177–181. https://doi.org/10.1007/s00467-018-4095-z
Colucci M, Carsetti R, Cascioli S et al (2016) B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol 27:1811–1822. https://doi.org/10.1681/ASN.2015050523
Dossier C, Jamin A, Deschênes G (2017) Idiopathic nephrotic syndrome: the EBV hypothesis. Pediatr Res 81:233–239
Jamin A, Berthelot L, Couderc A et al (2018) Autoantibodies against podocytic UCHL1 are associated with idiopathic nephrotic syndrome relapses and induce proteinuria in mice. J Autoimmun 89:149–161. https://doi.org/10.1016/j.jaut.2017.12.014
Lombel RM, Gipson DS (2012) Hodson EM (2012) Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol 283(28):415–426. https://doi.org/10.1007/S00467-012-2310-X
Rovin BH, Adler SG, Barratt J et al (2021) Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int 100:753–779. https://doi.org/10.1016/J.KINT.2021.05.015
Trautmann A, Vivarelli M, Samuel S et al (2020) IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 35:1529. https://doi.org/10.1007/S00467-020-04519-1
Franco LM, Gadkari M, Howe KN et al (2019) Immune regulation by glucocorticoids can be linked to cell type–dependent transcriptional responses. J Exp Med 216:384. https://doi.org/10.1084/JEM.20180595
Coutinho AE, Chapman KE (2011) The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335:2. https://doi.org/10.1016/J.MCE.2010.04.005
Tokunaga A, Sugiyama D, Maeda Y et al (2019) Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. J Exp Med 216:2701–2713. https://doi.org/10.1084/JEM.20190738
Van LF, Baus E, Smyth LA et al (2001) Glucocorticoids attenuate T cell receptor signaling. J Exp Med 193:803. https://doi.org/10.1084/JEM.193.7.803
Borel JF (1990) Mechanism of action and rationale for cyclosporin A in psoriasis. Br J Dermatol 122:5–12. https://doi.org/10.1111/J.1365-2133.1990.TB02876.X
Faul C, Donnelly M, Merscher-Gomez S et al (2008) The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14:931–938. https://doi.org/10.1038/nm.1857
Tsuda K, Yamanaka K, Kitagawa H et al (2012) Calcineurin inhibitors suppress cytokine production from memory T cells and differentiation of naïve T cells into cytokine-producing mature T cells. PLoS ONE 7:31465. https://doi.org/10.1371/JOURNAL.PONE.0031465
Heidt S, Roelen DL, Eijsink C et al (2010) Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin Exp Immunol 159:199–207. https://doi.org/10.1111/j.1365-2249.2009.04051.x
Hilchey SP, Palshikar MG, Emo JA et al (2020) Cyclosporine a directly affects human and mouse b cell migration in vitro by disrupting a hIF-1 αdependent, o2 sensing, molecular switch. BMC Immunol 21. https://doi.org/10.1186/s12865-020-0342-8
Allison A (2016) Mechanisms of action of mycophenolate mofetil: 14:2–8. https://doi.org/10.1191/0961203305LU2109OA
Mühlig AK, Lee JY, Kemper MJ et al (2019) Levamisole in children with idiopathic nephrotic syndrome: clinical efficacy and pathophysiological aspects. J Clin Med 8:860. https://doi.org/10.3390/JCM8060860
Karni A, Balashov K, Hancock W et al (2004) Cyclophosphamide modulates CD4+ T cells into a T helper type 2 phenotype and reverses increased IFN-gamma production of CD8+ T cells in secondary progressive multiple sclerosis. J Neuroimmunol 146:189–198. https://doi.org/10.1016/J.JNEUROIM.2003.10.036
Ghiringhelli F, Menard C, Puig P et al (2007) Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 56:641–648. https://doi.org/10.1007/S00262-006-0225-8
Yu C-C, Fornoni A, Weins A et al (2013) Abatacept in B7–1–positive proteinuric kidney disease. N Engl J Med 369:2416–2423. https://doi.org/10.1056/NEJMoa1304572
Pilot study to evaluate the safety and efficacy of abatacept in adults and children 6 years and older with excessive loss of protein in the urine due to either focal segmental glomerulosclerosis (FSGS) or minimal change disease (MCD) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02592798. Accessed 2 Oct 2021
Trachtman H, Gipson DS, Somers M et al (2018) Randomized clinical trial design to assess abatacept in resistant nephrotic syndrome. Kidney Int Reports 3:115–121. https://doi.org/10.1016/j.ekir.2017.08.013
Isom R, Shoor S, Higgins J et al (2019) Abatacept in steroid-dependent minimal change disease and CD80-uria. Kidney Int Reports 4:1349–1353. https://doi.org/10.1016/J.EKIR.2019.05.1155
Sawires H, Abdelaziz H, Ahmed HM et al (2019) Randomized controlled trial on immunomodulatory effects of azithromycin in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 34:1591–1597. https://doi.org/10.1007/s00467-019-04251-5
Greenbaum LA, Benndorf R, Smoyer WE (2012) Childhood nephrotic syndrome—current and future therapies. Nat Rev Nephrol 8:445–458. https://doi.org/10.1038/nrneph.2012.115
Oshima Y, Sumida K, Yamanouchi M et al (2020) Corticosteroid reduction by addition of cetirizine and montelukast in biopsy-proven minimal-change nephrotic syndrome concomitant with allergic disorders. Sci Rep 10. https://doi.org/10.1038/s41598-020-58463-z
Basu B (2014) Ofatumumab for rituximab-resistant nephrotic syndrome. 370:1268–1270. https://doi.org/10.1056/NEJMC1308488
Bernard J, Bruel A, Allain-Launay E et al (2018) Ofatumumab in post-transplantation recurrence of a pediatric steroid-resistant idiopathic nephrotic syndrome. Pediatr Transplant 22:e13175. https://doi.org/10.1111/PETR.13175
Bernard J, Lalieve F, Sarlat J et al (2020) Ofatumumab treatment for nephrotic syndrome recurrence after pediatric renal transplantation. Pediatr Nephrol 358(35):1499–1506. https://doi.org/10.1007/S00467-020-04567-7
Wang C-S, Liverman RS, Garro R et al (2017) Ofatumumab for the treatment of childhood nephrotic syndrome. Pediatr Nephrol 32:835–841. https://doi.org/10.1007/s00467-017-3621-8
Dossier C, Prim B, Moreau C et al (2021) A global antiB cell strategy combining obinutuzumab and daratumumab in severe pediatric nephrotic syndrome. Pediatr Nephrol 36:1. https://doi.org/10.1007/S00467-020-04811-0
Author information
Authors and Affiliations
Contributions
Conceptualization: Konstantina Kitsou, Vana Spoulou; Writing — original draft preparation: Konstantina Kitsou, Vana Spoulou; Writing — review and editing: Konstantina Kitsou, Varvara Askiti, Andromachi Mitsioni, Vana Spoulou; Supervision: Vana Spoulou.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Nicole Ritz
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
431_2021_4357_MOESM1_ESM.pdf
Online Resource 1 (PDF file): Studies on the immunological mechanisms of Idiopathic Nephrotic Syndrome, conducted in children, their population characteristics, immunological parameters assessed and study outcomes. (PDF 167 KB)
Rights and permissions
About this article
Cite this article
Kitsou, K., Askiti, V., Mitsioni, A. et al. The immunopathogenesis of idiopathic nephrotic syndrome: a narrative review of the literature. Eur J Pediatr 181, 1395–1404 (2022). https://doi.org/10.1007/s00431-021-04357-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04357-9