Abstract
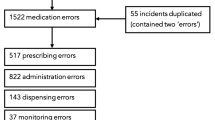
The aim was to describe medication errors (MEs) in hospitalized children reported to the national mandatory reporting and learning system, the Danish Patient Safety Database (DPSD). MEs were extracted from DPSD from the 5-year period of 2010–2014. We included reports from public hospitals on patients aged 0–17 years and categorized by reporters as medication-related. Reports from psychiatric wards and outpatient clinics were excluded. A ME was defined as any medication-related error occurring in the medication process whether harmful or not. MEs were categorized as harmful if they resulted in actual harm or interventions to prevent harm. MEs were further categorized according to occurrence in the medication process, type of error, and the medicines involved. A total of 2071 MEs including 487 harmful MEs were identified. Most MEs occurred during prescribing (40.8%), followed by dispensing (38.7%). Harmful MEs occurred mainly during dispensing (40.3%). Dosing errors were the most reported type of error, 47.7% of all MEs and 45.4% of harmful MEs. Antibiotics and analgesics were the most frequently reported medication classes. Common medicines associated with MEs included morphine, paracetamol, and gentamicin. MEs caused no harm (74.9%), mild (11.7%), moderate (10.5%), or severe harm (1.3%), but none were lethal.
Conclusion: MEs in hospitalized children occur in all medication processes and mainly involve dosing errors. Strategies should be developed to prevent MEs as these still threaten medication safety in pediatric inpatients.
What is known: • Hospitalized children are more likely to experience medication errors than adults. • Voluntary national and local reporting and learning systems have previously been used to describe the nature and types of medication errors. |
What is new: • Medication errors in hospitalized children occur in all steps of the medication process, most frequently involving dosing errors and most commonly involving morphine, paracetamol, and gentamicin. • Of the medication errors, 1.3% cause severe harm, but no fatal errors were reported. |


Similar content being viewed by others
Abbreviations
- ATC:
-
Anatomical Therapeutic Chemical Classification System
- DPSA:
-
Danish Patient Safety Authority
- DPSD:
-
Danish Patient Safety Database
- ME:
-
medication errors
- NCC MERP:
-
The National Coordinating Council for Medication Error Reporting and Prevention
- WHO:
-
World Health Organization
References
Arabi YM, Al Owais SM, Al-Attas K et al (2016) Learning from defects using a comprehensive management system for incident reports in critical care. Anaesth Intensive Care 44:210–220
Aspden P, Wolcott JA, Bootman JL, Cronenwett LR (2006) Committee on identifying and preventing medication errors: preventing medication errors. The National Academies Press, Washington
Belela ASC, Peterlini MAS, Pedreira MLG (2011) Medication errors reported in a pediatric intensive care unit for oncologic patients. Cancer Nurs 34:393–400. https://doi.org/10.1097/NCC.0b013e3182064a6a
Chua SS, Chua HM, Omar A (2010) Drug administration errors in paediatric wards: a direct observation approach. Eur J Pediatr 169:603–611. https://doi.org/10.1007/s00431-009-1084-z
Classen DC, Pestotnik SL, Evans RS, Burke JP (2005) Computerized surveillance of adverse drug events in hospital patients. 1991. Qual Saf Health Care 14:221–225; discussion 225-226. https://doi.org/10.1136/qshc.2002.002972/10.1136/qshc.2005.014522
Evans SM, Berry JG, Smith BJ et al (2006) Attitudes and barriers to incident reporting: a collaborative hospital study. Qual Saf Health Care 15:39–43. https://doi.org/10.1136/qshc.2004.012559
Farley DO, Haviland A, Champagne S et al (2008) Adverse-event-reporting practices by US hospitals: results of a national survey. Qual Saf Health Care 17:416–423. https://doi.org/10.1136/qshc.2007.024638
Fernández-Llamazares CM, Pozas M, Feal B et al (2013) Profile of prescribing errors detected by clinical pharmacists in paediatric hospitals in Spain. Int J Clin Pharm 35:638–646. https://doi.org/10.1007/s11096-013-9785-9
Ghaleb MA, Barber N, Franklin BD et al (2006) Systematic review of medication errors in pediatric patients. Ann Pharmacother 40:1766–1776. https://doi.org/10.1345/aph.1G717
Ghaleb MA, Barber N, Franklin BD, Wong ICK (2010) The incidence and nature of prescribing and medication administration errors in paediatric inpatients. Arch Dis Child 95:113–118. https://doi.org/10.1136/adc.2009.158485
Holdsworth MT, Fichtl RE, Raisch DW et al (2007) Impact of computerized prescriber order entry on the incidence of adverse drug events in pediatric inpatients. Pediatrics 120:1058–1066. https://doi.org/10.1542/peds.2006-3160
Hoonhout LHF, de Bruijne MC, Wagner C et al (2010) Nature, occurrence and consequences of medication-related adverse events during hospitalization: a retrospective chart review in the Netherlands. Drug Saf 33:853–864. https://doi.org/10.2165/11536800-000000000-00000
Hughes RG, Edgerton EA (2005) Reducing pediatric medication errors: children are especially at risk for medication errors. Am J Nurs 105:79–80 82, 85 passim
Indenrigs- og Sundhedsministeriet (2006) Evaluering af lov om patientsikkerhed http://www.sum.dk/Aktuelt/Nyheder/Patienters-retstilling/2006/September/Evaluering_om_lov.aspx. Accessed 13 June 2016
Institute for Safe Medication Practices (2014) ISMP high-alert medications. https://www.ismp.org/tools/highalertmedicationLists.asp. Accessed 02 May 2017
Kaushal R, Bates DW, Landrigan C et al (2001) Medication errors and adverse drug events in pediatric inpatients. JAMA 285:2114–2120
Kohn LT, Corrigan JM, Donaldson MS (2000) To err is human: building a safer health system. National Academy Press, Washington
Kunac DL, Kennedy J, Austin N, Reith D (2009) Incidence, preventability, and impact of adverse drug events (ADEs) and potential ADEs in hospitalized children in New Zealand: a prospective observational cohort study. Paediatr Drugs 11:153–160
Maaskant JM, Eskes A, van Rijn-Bikker P et al (2013) High-alert medications for pediatric patients: an international modified Delphi study. Expert Opin Drug Saf 12:805–814. https://doi.org/10.1517/14740338.2013.825247
Manias E, Kinney S, Cranswick N, Williams A (2014) Medication errors in hospitalised children. J Paediatr Child Health 50:71–77. https://doi.org/10.1111/jpc.12412
Persondataloven - Lov om behandling af personoplysninger (2000) Retsinformation. https://www.retsinformation.dk/forms/r0710.aspx?id=828. Accessed 02 May 2017
Pronovost PJ, Morlock LL, Sexton JB et al (2008) Improving the Value of Patient Safety Reporting Systems. In: Henriksen K, Battles JB, Keyes MA, Grady ML (2008) Advances in Patient Safety: New Directions and Alternative Approaches (Vol 1: Assessment). Rockville (MD): Agency for Healthcare Research and Quality. http://www.ncbi.nlm.nih.gov/books/NBK43621/. Accessed 02 May 2017
Rashed AN, Tomlin S, Aguado V et al (2016) Sources and magnitude of error in preparing morphine infusions for nurse-patient controlled analgesia in a UK paediatric hospital. Int J Clin Pharm. https://doi.org/10.1007/s11096-016-0369-3
Richey RH, Shah UU, Peak M et al (2013) Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr 13:81. https://doi.org/10.1186/1471-2431-13-81
Snyder RA, Fields W (2010) A model for medication safety event detection. Int J Qual Health Care J Int Soc Qual Health Care ISQua 22:179–186. https://doi.org/10.1093/intqhc/mzq014
Taylor JA, Brownstein D, Christakis DA et al (2004) Use of incident reports by physicians and nurses to document medical errors in pediatric patients. Pediatrics 114:729–735. https://doi.org/10.1542/peds.2003-1124-L
The National Coordinating Council for Medication Error Reporting and Prevention (NCC-MERP) (2017) About medication errors. http://www.nccmerp.org/about-medication-errors. Accessed 02 May 2017
The National Coordinating Council for Medication Error Reporting and Prevention (NCC-MERP) (1998) NCC MERP taxonomy of medication errors. http://www.nccmerp.org/taxonomy-medication-errors-now-available. Accessed 02 May 2017
Tundia NL, Heaton PC, Kelton CML (2011) The national burden of E-code-identified adverse drug events among hospitalized children using a national discharge database. Pharmacoepidemiol Drug Saf 20:866–878. https://doi.org/10.1002/pds.2150
Vincent C, Stanhope N, Crowley-Murphy M (1999) Reasons for not reporting adverse incidents: an empirical study. J Eval Clin Pract 5:13–21
Wang JK, Herzog NS, Kaushal R et al (2007) Prevention of pediatric medication errors by hospital pharmacists and the potential benefit of computerized physician order entry. Pediatrics 119:e77–e85. https://doi.org/10.1542/peds.2006-0034
WHO collaborating Centre for Drug Statistics Methodology Anatomical therapeutic chemical (ATC) classification index with defined daily doses (DDD). https://www.whocc.no/. Accessed 02 May 2017
Williams SD, Ashcroft DM (2009) Medication errors: how reliable are the severity ratings reported to the national reporting and learning system? Int J Qual Health Care J Int Soc Qual Health Care ISQua 21:316–320. https://doi.org/10.1093/intqhc/mzp034
Wilson DG, McArtney RG, Newcombe RG et al (1998) Medication errors in paediatric practice: insights from a continuous quality improvement approach. Eur J Pediatr 157:769–774
Wong ICK, Ghaleb MA, Franklin BD, Barber N (2004) Incidence and nature of dosing errors in paediatric medications: a systematic review. Drug Saf 27:661–670
World Health Organization (1972) Technical Report No 498: International drug monitoring: the role of national centres https://www.who-umc.org/media/2680/who-technical-report-498.pdf. Accessed 02 May 2017
Acknowledgements
We wish to thank the Division for Learning at the Danish Patient Safety Authority for their contribution to this study. Thank you for your help and support making the data from the Danish Patient Safety Database available to us.
Funding
This study was funded by Amgros I/S, The Hospital Pharmacies and Amgros’ Research and Development Fund, Copenhagen, Denmark, and the Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark. The organizations sponsored the PhD scholarship of Rikke Rishoej. Lene Juel Kjeldsen is employed by Amgros I/S.
Author information
Authors and Affiliations
Contributions
RMR conceptualized and designed the study and drafted the initial manuscript and was responsible for data collection and initial analysis.LJK, HBTC, ABA, and JH contributed to the conceptualization of the study, data analysis, and interpretation of data and critically reviewed the manuscript. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Conflict of interest
Rikke Rishoej received grants from Pfizer to attend the 23rd Congress of European Association of Hospital Pharmacists in Vienna in 2016. Anna Birna Almarsdóttir declares that she has no conflict of interest. Henrik Boye Thybo Christesen declares that he has no conflict of interest. Jesper Hallas declares that he has no conflict of interest. Lene Juel Kjeldsen declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Nicole Ritz
Rights and permissions
About this article
Cite this article
Rishoej, R.M., Almarsdóttir, A.B., Christesen, H.T. et al. Medication errors in pediatric inpatients: a study based on a national mandatory reporting system. Eur J Pediatr 176, 1697–1705 (2017). https://doi.org/10.1007/s00431-017-3023-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-017-3023-8