Abstract
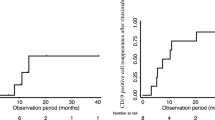
The severity and duration of immunosuppression caused by corticosteroids (CSs) usage have not been extensively studied. We aimed to investigate the effects of CSs on the various compartments of immune system in relation to timing of initiation and persistence of therapy. Pediatric patients with idiopathic nephrotic syndrome (NS) treated with 2 mg/kg/day prednisolone and healthy control (HC) were enrolled. Blood samples were drawn for immunologic analyses at baseline and at the first and second weeks and first, second, and third months of CS therapy in addition to first and second weeks and first, second, and third months of discontinuation. Fourteen patients (M/F, 7/7) between 1 and 8 years old were evaluated. Untreated NS exhibited high absolute lymphocyte count (ALC)(p = 0.010), absolute CD3+ T cells (p = 0.020) and absolute CD8+ T cells (p = 0.006) compared to HC. Suppression in ALC was observed and nadir value was noted at first month of therapy compared to baseline (p = 0.002). The CD4+ (p = 0.036) and CD8+ T cell (p = 0.013) counts decreased significantly at the first week of treatment compared to baseline. While baseline B cell counts was indifferent from HC, gradually increased in 2 weeks of CS initiation and decreased during the treatment with a statistical significance compared to HC (p = 0.010). However, after cessation of CS, B cell counts continued to decline and found to be significantly different than baseline at first week (p = 0.008) and at third month (p = 0.040).
Conclusion: Apart from baseline lymphocyte subset changing observed in untreated NS patients, our data implies that T cells were suppressed very early in the CS treatment. Interestingly, depressed B cell counts were detected later but persisted even after CS cessation. Due to early decrease in T cells, it would be beneficial to assume the patients as immunosuppressed at the very beginning of CS treatment to avoid infections.
What is Known: |
• Corticosteroids (CSs) are widely used for a variety of diseases including nephrotic syndrome, which is related with complex immune disturbance including T and B cells dysfunctions. |
• CSs induce neutrophilic leukocytosis concomitant with lymphopenia and eosinopenia leading to immunosupression. |
What is New: |
• T cell subsets and proliferation are susceptible to CSs more than B cells; however, the reversibility is faster with dose reduction in CS. |
• The change of B cells and B cell subtypes (CD27 + memory) shows prolonged effect of CSs on B cells which may alter antibody production even after 3 months of CSs cessation. |



Similar content being viewed by others
Abbreviations
- CS:
-
Corticosteroid
- GR:
-
Glucocorticoid receptor
- NS:
-
Nephrotic syndrome
- HC:
-
Healthy control
- CFSE:
-
Carboxyfluorescein diacetate, succinimidyl ester
- PBMC:
-
Peripheral Blood Mononuclear Cells
- WBC:
-
White blood cell
- ALC:
-
Absolute lymphocyte count
- ANC:
-
Absolute neutrophil count
- CD4+ :
-
Helper T cells
- CD8+ :
-
Cytotoxic T cells
References
Ashwell JD, Lu FW, Vacchio MS (2000) Glucocorticoids in T cell development and function. Ann Rev Immunol 18:309–345
Baker C, Pickering L, Chilton L et al (2011) General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 60:1–64
Chatham WW, Kimberly RP (2001) Treatment of lupus with corticosteroids. Lupus 10:140–147
Coutinho AE, Chapman KE (2011) The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335:2–13. doi:10.1016/j.mce.2010.04.005
Cupps TR, Gerrard TL, Falkoff RJ, Whalen G, Fauci AS (1985) Effects of in vitro corticosteroids on B cell activation, proliferation, and differentiation. J Clin Invest 75:754–761
Fan PT, Yu DT, Clements PJ, Fowlston S, Eisman J, Bluestone R (1978) Effect of corticosteroids on the human immune response: comparison of one and three daily 1 gm intravenous pulses of methylprednisolone. J Lab Clin Med 91:625–634
Fauci AS, Dale DC, Balow JE (1976) Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med 84:304–315
Fedor ME, Rubinstein A (2006) Effects of long-term low-dose corticosteroid therapy on humoral immunity. Ann Allergy Asthma Immunol 97:113–116
Fiser RT, Arnold WC, Charlton RK, Steele RW, Childress SH, Shirkey B (1991) T-lymphocyte subsets in nephrotic syndrome. Kidney Int 40:913–916
Flammer JR, Rogatsky I (2011) Minireview: glucocorticoids in autoimmunity: unexpected targets and mechanisms. Mol Endocrinol 25:1075–1086. doi:10.1210/me.2011-0068
Giangiacomo J, Cleary TG, Cole BR, Hoffstein P, Robson AM (1975) Serum immunoglobulins in the nephrotic syndrome. A possible cause of minimal change nephrotic syndrome. N Engl J Med 293:08–12
Gipson DS, Massengill SF, Yao L et al (2009) Management of childhood onset nephrotic syndrome. Pediatrics 124:747–757. doi:10.1542/peds.2008-1559
Guigonis V, Dallocchio A, Baudouin V et al (2008) Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol 23:1269–1279
Hulton SA, Shah V, Byrne MR, Morgan G, Barratt TM, Dillon MJ (1994) Lymphocyte subpopulations, interleukin-2 and interleukin-2 receptor expression in childhood nephrotic syndrome. Pediatr Nephrol 8:135–139
Iharada A, Kaneko K, Tsuji S, Hasui M, Kanda S, Nishiyama T (2009) Increased nitric oxide production by T- and B-cells in idiopathic nephrotic syndrome. Pediatr Nephrol 24:1033–1038. doi:10.1007/s00467-008-1092-7
Kanai T, Shiraishi H, Yamagata T et al (2010) Th2 cells predominate in idiopathic steroid-sensitive nephrotic syndrome. Clin Exp Nephrol 14:578–583
Kaneko K, Tsuji S, Kimata T, Kitao T, Yamanouchi S, Kato S (2015) Pathogenesis of childhood idiopathic nephrotic syndrome: a paradigm shift from T-cells to podocytes. World J Pediatr 11:21–28. doi:10.1007/s12519-015-0003-9
Kelly ME, Juern AM, Grossman WJ, Schauer DW, Drolet BA (2010) Immunosuppressive effects in infants treated with corticosteroids for infantile hemangiomas. Arch Dermatol 146:767–774. doi:10.1001/archdermatol.2010.90
Kemper MJ, Zepf K, Klaassen I, Link A, Muller-Wiefel DE (2005) Changes of lymphocyte populations in pediatric steroid-sensitive nephrotic syndrome are more pronounced in remission than in relapse. Am J Nephrol 25:132–137
Lanza L, Scudeletti M, Puppo F et al (1996) Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin Exp Immunol 103:482–490
Leussink VI, Jung S, Merschdorf U, Toyka KV, Gold R (2001) High-dose methylprednisolone therapy in multiple sclerosis induces apoptosis in peripheral blood leukocytes. Arch Neurol 58:91–97
Lombel RM, Gipson DS, Hodson EM (2013) Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol 28:415–426. doi:10.1007/s00467-012-2310-x
Meagher LC, Cousin JM, Seckl JR, Haslett C (1966) Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol 156:4422–4428
Nijhuis EW, Hinloopen B, van Lier RA, Nagelkerken L (1995) Differential sensitivity of human naive and memory CD4+ T cells for dexamethasone. Int Immunol 7:591–595
Nilganuwonge S, Harisdangkul V, Rockhold L, Lewis RE, Cruse JM (1988) Lymphocyte subset T4/T8 ratio in systemic lupus erythematosus: correlation with disease activity, laboratory abnormalities and treatment. Asian Pac J Allergy Immunol 6:23–28
Pereira Wde F, Brito Melo GE, Guimarães FT, Carvalho TG, Mateo EC, Simões E, Silva AC (2014) The role of the immune system in idiopathic nephrotic syndrome: a review of clinical and experimental studies. Inflamm Res 63:1–12
Shalhoub RJ (1974) Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet 2:556–560.22
Tornatore KM, Reed K, Venuto R (1995) 24-hour immunologic assessment of CD4+ and CD8+ lymphocytes in renal transplant recipients receiving chronic methylprednisolone. Clin Nephrol 44:290–298
Van der Berg PJ, Hoevenaars EC, Yong SL et al (2012) Circulating lymphocyte subsets in different clinical situations after renal transplantation. Immunology 136:198–207. doi:10.1111/j.1365-2567.2012.03570.x
Yildiz B, Cetin N, Kural N, Colak O (2013) CD19 + CD23+ B cells, CD4 + CD25+ T cells, E-selectin and interleukin-12 levels in children with steroid sensitive nephrotic syndrome. Ital J Pediatr 39:42. doi:10.1186/1824-7288-39-42
Younger RE, Gerber PS, Herrod HG, Cohen RM, Crawford LV (1987) Intravenous methylprednisolone efficacy in status asthmaticus of childhood. Pediatrics 80:225–230
Authors’ contributions
HEB followed up patients, data collection and analysis, and drafting of the manuscript.
SB had substantial contributions to the data analysis and drafting of the manuscript.
EKA had participated in writing of the manuscript.
IG followed up patients, data collection, and commented on the manuscript draft.
NY followed up patients and commented on the manuscript draft.
DC performed flow cytometer analysis.
IO performed the lymphocyte proliferation studies and participated in writing of the manuscript.
AO had substantial contribution to the conception and commented on the manuscript draft.
HA had substantial contribution to the conception and commented on the manuscript draft.
IB supervised the study, had substantial contribution to the conception, and commented on the manuscript draft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the local ethics committee of Marmara University (IRB number: 00009067); all procedures were in accordance with the 1964 Helsinki declaration, and a written informed consent was obtained from all parents. Due to the young age of our patients, a simple oral description of the study was given to participating child in the presence of their parent(s) and a verbal assent was requested.
Funding
No sources of financial and material support to be declared.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Marmara University and with the 1964 Helsinki declaration.
Informed consent
Informed consent was obtained for the parents of infants included in the study.
Additional information
Communicated by David Nadal
Revisions received: 18 November 2015; 9 January 2016
Rights and permissions
About this article
Cite this article
Baris, H.E., Baris, S., Karakoc-Aydiner, E. et al. The effect of systemic corticosteroids on the innate and adaptive immune system in children with steroid responsive nephrotic syndrome. Eur J Pediatr 175, 685–693 (2016). https://doi.org/10.1007/s00431-016-2694-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-016-2694-x