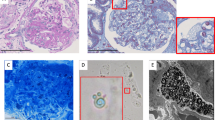
Abstract
Fabry disease is an X-linked glycosphingolipidosis caused by deficient synthesis of the enzyme α-galactosidase A, which results in accumulations of globotriaosylceramide (GL-3) in systemic tissues. Nephropathy is a dominant feature of Fabry disease. It still remains unclear how the nephropathy progresses. Recombinant agalsidase replacement therapy is currently the only approved, specific therapy for Fabry disease. The optimal dose of replacement enzyme also still remains unclear. The worldwide shortage of agalsidase-β in 2009 forced dose reduction of administration. It showed that the proteinuria emerged like surges, followed by temporary plasma GL-3 elevations in the early stages of classic Fabry disease. Additionally, it also showed that 1 mg/kg of agalsidase-β every other week could clear the GL-3 accumulations from podocytes and was required to maintain negative proteinuria and normal plasma GL-3 levels.
Conclusion: This observation of a young patient with classic Fabry disease about 5 years reveals that the long-term, low-dose agalsidase-β caused proteinuria surges, but not persistent proteinuria, followed by temporary plasma GL-3 elevations, and agalsidase-β at 1 mg/kg every other week could clear accumulated GL-3 from podocytes and was required to maintain normal urinalysis and plasma GL-3 levels.
What is Known: • Patients with Fabry disease show GL-3 accumulations in podocytes and foot process effacement without proteinuria. • The optimal dose of replacement enzyme (agalsidase-α or -β) remains unclear. |
What is New: • The long-term, low-dose agalsidase-β caused proteinuria surge, but not persistent proteinuria, followed by temporary plasma GL-3 elevations • Agalsidase-β at 1 mg/kg every other week could clear accumulated GL-3 from podocytes, and was required to maintain normal urinalysis and normal plasma GL-3 levels. |


Similar content being viewed by others
Abbreviations
- EOW:
-
Every other week
- GL-3:
-
Globotriaosylceramide
- UCr:
-
Urinary creatinine
- UP:
-
Urinary protein
References
Germain DP, Waldek S, Banikazemi M, Bushinsky DA, Charrow J, Desnick RJ, Lee P, Loew T, Vedder AC, Abichandani R, Wilcox WR, Guffon N (2007) Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 18:1547–1557
Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR (2015) Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet 52:353–358
Kanai T, Yamagata T, Ito T, Odaka J, Saito T, Aoyagi J, Kobayashi M, Ohashi T, Ueda Y, Momoi MY (2011) Foot process effacement with normal urinalysis in classic Fabry disease. JIMD Rep 1:39–42
Kawachi H, Han GD, Miyauchi N, Hashimoto T, Suzuki K, Shimizu F (2009) Therapeutic targets in the podocyte: findings in anti-slit diaphragm antibody-induced nephropathy. J Nephrol 22:450–456
Lubanda JC, Anijalg E, Bzduch V, Thurberg BL, Benichou B, Tylki-Szymanska A (2009) Evaluation of a low dose, after a standard therapeutic dose, of agalsidase beta during enzyme replacement therapy in patients with Fabry disease. Genet Med 11:256–264
Smid BE, Rombach SM, Aerts JM, Kuiper S, Mirzaian M, Overkleeft HS, Poorthuis BJ, Hollak CE, Groener JE, Linthorst GE (2011) Consequences of a global enzyme shortage of agalsidase beta in adult Dutch Fabry patients. Orphanet J Rare Dis 6:69
Tondel C, Bostad L, Larsen KK, Hirth A, Vikse BE, Houge G, Svarstad E (2013) Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol 24:137–148
Tondel C, Kanai T, Larsen KK, Ito S, Politei JM, Warnock DG, Svarstad E (2015) Foot process effacement is an early marker of nephropathy in young classic Fabry patients without albuminuria. Nephron Physiol 129:16–21
Vedder AC, Linthorst GE, Houge G, Groener JE, Ormel EE, Bouma BJ, Aerts JM, Hirth A, Hollak CE (2007) Treatment of Fabry disease: outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS One 2:e598
Wang L, Tang Y, Howell DN, Ruiz P, Spurney RF (2012) A novel mouse model of podocyte depletion. Nephron Exp Nephrol 121:e10–e22
Warnock DG, Mauer M (2014) Fabry disease: dose matters. J Am Soc Nephrol 25:653–655
Wiggins J (2009) Podocytes and glomerular function with aging. Semin Nephrol 29:587–593
Author’s contributions
Dr. Kanai was the author of this manuscript and the physician in charge of the patient; Prof. Yamagata acted as an advisor for this study; Dr. Ito, Dr. Odaka, Dr. Saito, Dr. Aoyagi, and Dr. Betsui assisted in treating the patient.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of financial potential conflicts of interest
Dr. Kanai received speaking fee, ¥ 500,00 yen, on “Foot process effacement with normal urinalysis in classic Fabry disease. JIMD reports 1:39-42” from Genzyme co. Other authors declare no financial relationships that could be relevant to this work.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Ethical approval
All applicable national and institutional guidelines for the care were followed. All procedures performed in this study were accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Witten informed consent was obtained from the patient and his parent.
Additional information
Communicated by Beat Steinmann
This manuscript has not been published and is not under consideration for publication elsewhere. All the authors have read the manuscript and have approved this submission.
Rights and permissions
About this article
Cite this article
Kanai, T., Ito, T., Odaka, J. et al. Surges in proteinuria are associated with plasma GL-3 elevations in a young patient with classic Fabry disease. Eur J Pediatr 175, 427–431 (2016). https://doi.org/10.1007/s00431-015-2646-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2646-x