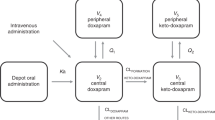
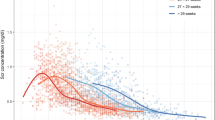
Abstract
This study aimed to determine the population pharmacokinetics of doxapram in low-birth-weight (LBW) infants. A total of 92 serum concentration measurements that were obtained from 34 Japanese neonates were analyzed using nonlinear mixed-effect modeling (NONMEM). Estimates generated by NONMEM indicated that clearance of doxapram (CL; L/kg/h) was affected by postmenstrual age (PMA; weeks), body weight (BW; g), and aspartate aminotransferase (AST; IU/L). In addition, the volume of distribution (Vd; L/kg) was affected by gestational age (GA; weeks). The final pharmacokinetic model was as follows: CL = BW / PMA × 0.0453 × serum AST−0.373; Vd = 2.54 (if GA >28 weeks) and Vd = 2.54 × 2.11 (if GA ≤28 weeks). The interindividual variabilities in CL and Vd were 39.9 and 83.0 %, respectively, and the residual variability was 20.9 %. To clarify the reasons for large interindividual variations, the enzymes involved in the metabolic pathway of doxapram were also determined. We found that doxapram was metabolized by CYP3A4/5.
Conclusion: We report the population pharmacokinetics of doxapram in neonates and the involvement of CYP3A4/5 in its metabolism. The final model of population pharmacokinetics may be useful for formulating a safe and effective dosage regimen and for predicting serum doxapram concentrations in neonates.




Similar content being viewed by others
Abbreviations
- AOP:
-
Apnea of prematurity
- AST:
-
Aspartate aminotransferase
- BW:
-
Body weight
- CL:
-
Clearance
- GA:
-
Gestational age
- HLM:
-
Human liver microsomes
- HPLC:
-
High-performance liquid chromatography
- LBW infants:
-
Low-birth-weight infants
- MAE:
-
Mean absolute error
- ME:
-
Mean prediction error
- MS:
-
Mass spectrometry
- NONMEM:
-
Nonlinear mixed-effect modeling
- PMA:
-
Postmenstrual age
- PNA:
-
Postnatal age
- OBJ:
-
Objective function
- Vd:
-
Volume of distribution
References
Alden ER, Mandelkorn T, Woodrum DE, Wennberg RP, Parks CR, Hodson WA (1972) Morbidity and mortality of infants weighing less than 1,000 grams in an intensive care nursery. Pediatrics 50:40–49
Allegaert K, Anderson BJ, van den Anker JN, Vanhaesebrouck S, de Zegher F (2007) Renal drug clearance in preterm neonates: relation to prenatal growth. Ther Drug Monit 29:284–291
Alpan G, Eyal F, Sagi E, Springer C, Patz D, Goder K (1984) Doxapram in the treatment of idiopathic apnea of prematurity unresponsive to aminophylline. J Pediatr 104:634–637
Anderson BJ, Allegaert K, Holford NH (2006) Population clinical pharmacology of children: general principles. Eur J Pediatr 165:741–746
Aranda JV, Beharry K, Rex J, Linder N, Blanchard P (1988) High pressure liquid chromatographic microassay for simultaneous measurement of doxapram and its metabolites in premature newborn infants. J Liq Chromatogr 11:2983–2991
Bairam A, Blanchard PW, Mullahoo K, Beharry K, Laudignon N, Aranda JV (1990) Pharmacodynamic effects and pharmacokinetic profiles of keto-doxapram and doxapram in newborn lambs. Pediatr Res 28:142–146
Bairam A, Branchaud C, Beharry K, Rex J, Laudignon N, Papageorgiou A, Aranda JV (1991) Doxapram metabolism in human fetal hepatic organ culture. Clin Pharmacol Ther 50:32–38
Barbé F, Hansen C, Badonnel Y, Legagneur H, Vert P, Boutroy MJ (1999) Severe side effects and drug plasma concentrations in preterm infants treated with doxapram. Ther Drug Monit 21:547–552
Barrington KJ, Finer NN, Torok-Both G, Jamali F, Coutts RT (1987) Dose–response relationship of doxapram in the therapy for refractory idiopathic apnea of prematurity. Pediatrics 80:22–27
Beaudry MA, Bradley JM, Gramlich LM, LeGatt D (1988) Pharmacokinetics of doxapram in idiopathic apnea of prematurity. Dev Pharmacol Ther 11:65–72
Brion LP, Vega-Rich C, Reinersman G, Roth P (1991) Low-dose doxapram for apnea unresponsive to aminophylline in very low birthweight infants. J Perinatol 11:359–364
Coutts RT, Jamali F, Malek F, Peliowski A, Finer NN (1991) Urinary metabolites of doxapram in premature neonates. Xenobiotica 21:1407–1418
Eyal F, Alpan G, Sagi E, Glick B, Peleg O, Dgani Y, Arad I (1985) Aminophylline versus doxapram in idiopathic apnea of prematurity: a double-blind controlled study. Pediatrics 75:709–713
Fukuda T, Yukawa E, Kondo G, Maeda T, Shin-o T, Kondo Y, Imamura T, Irikura M, Irie T (2005) Population pharmacokinetics of theophylline in very premature Japanese infants with apnoea. J Clin Pharm Ther 30:591–596
Hayakawa F, Hakamada S, Kuno K, Nakashima T, Miyachi Y (1986) Doxapram in the treatment of idiopathic apnea of prematurity: desirable dosage and serum concentrations. J Pediatr 109:138–140
Higuchi S, Aoyama T, Horioka M (1987) PEDA: a microcomputer program for parameter estimation and dosage adjustment in clinical practice. J Pharmacobiodyn 10:703–718
Hines RN (2007) Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol 21:169–175
Jamali F, Barrington KJ, Finer NN, Coutts RT, Torok-Both GA (1988) Doxapram dosage regimen in apnea of prematurity based on pharmacokinetic data. Dev Pharmacol Ther 11:253–257
Jamali F, Coutts RT, Malek F, Finer NN, Peliowski A (1991) Lack of a pharmacokinetic interaction between doxapram and theophylline in apnea of prematurity. Dev Pharmacol Ther 16:78–82
Kumita H, Mizuno S, Shinohara M, Ichikawa T, Yamazaki T (1991) Low-dose doxapram therapy in premature infants and its CSF and serum concentrations. Acta Paediatr Scand 80:786–791
Lee TC, Charles B, Steer P, Flenady V, Shearman A (1997) Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clin Pharmacol Ther 61:628–640
Mohangoo AD, Blondel B, Gissler M, Velebil P, Macfarlane A, Zeitlin J, Committee E-PS (2013) International comparisons of fetal and neonatal mortality rates in high-income countries: should exclusion thresholds be based on birth weight or gestational age? PLoS One 8:e64869
Ogawa Y, Irikura M, Tomiyasu M, Uryu M, Ishitsuka Y, Kondo Y, Yamazaki T, Yukawa E, Irie T (2013) Dosage regimen of low-dose doxapram for treatment of low-birth weight infants with idiopathic apnea of prematurity. Japan J Dev Pharmacol Ther 26:97–101
Pediatrics CoFaNAAo (2003) Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 111:914–917
Peliowski A, Finer NN (1990) A blinded, randomized, placebo-controlled trial to compare theophylline and doxapram for the treatment of apnea of prematurity. J Pediatr 116:648–653
Prins SA, Pans SJ, van Weissenbruch MM, Walther FJ, Simons SH (2013) Doxapram use for apnoea of prematurity in neonatal intensive care. Int J Pediatr 2013:251047
Robinson D, Weiner CP, Nakamura KT, Robillard JE (1990) Effect of intrauterine growth retardation on renal function on day one of life. Am J Perinatol 7:343–346
Robson RH, Prescott LF (1979) A pharmacokinetic study of doxapram in patients and volunteers. Br J Clin Pharmacol 7:81–87
Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV, Davis PG, Tin W, Moddemann D, Solimano A, Ohlsson A, Barrington KJ, Roberts RS, Investigators CAPCT (2012) Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 307:275–282
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W, Group CfAoPT (2007) Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 357:1893–1902
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512
Silver LE, Decamps PJ, Korst LM, Platt LD, Castro L (2003) Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am J Obstet Gynecol 188:1320–1325
Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, Zaya MJ (2003) Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther 307:573–582
Tay-Uyboco J, Kwiatkowski K, Cates DB, Seifert B, Hasan SU, Rigatto H (1991) Clinical and physiological responses to prolonged nasogastric administration of doxapram for apnea of prematurity. Biol Neonate 59:190–200
Victor S, Dickinson H, Turner MA (2011) Plasma aminotransferase concentrations in preterm infants. Arch Dis Child Fetal Neonatal Ed 96:F144–F145
Yamazaki T (2013) The efficacy of doxapram for apnea in low birth weight infants. Multi-center, randomized, double-blind, controlled trial by neonatal research network. Perinat Med 43:603–610
Acknowledgments
This study was supported by a grant-in-aid for scientific research from the Ministry of Health, Labour and Welfare (Research Project Number: 23590189) and by the doxapram group of the Neonatal Research Network. The following investigators and research staff (no particular order) contributed to the trial. Fujita Health University, Aichi, Japan: Toshio Yamazaki, Tadayoshi Hata, Shuji Hashimoto, and Hiroko Mizutani; Osaka Medical Center and Research Institute for Maternal and Child Health, Osaka, Japan: Hiroyuki Kitajima, Masanori Fujimura, and Shinya Hirano; Kumamoto University, Kumamoto, Japan: Tetsumi Irie and Mitsuru Irikura; Kakogawa City Hospital, Hyogo, Japan: Akihito Ishida and Masanori Murase; Toyohashi Municipal Hospital, Aichi, Japan: Norihisa Koyama; Hiroshima City Hospital, Hiroshima, Japan: Michiko Hayashidani; Shiga University of Medical Science, Shiga, Japan: Hirofumi Aotani; National Center for Child Health and Development, Tokyo, Japan: Hidefumi Nakamura; Kagawa University, Kagawa, Japan: Susumu Itoh; and Kissei Pharmaceutical Co., Ltd., Nagano, Japan: Mutsuo Kanzawa. Doxapram used in this study was donated by Kissei Pharmaceutical Co. Ltd.
Ethical standards
This study was approved by the ethics committee at the each institution. Parents of eligible LBW infants gave written informed consent.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Patrick Van Reempts
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(XLSX 13 kb)
Online Resource 2
Assay procedure of doxapram and keto-doxapram. (PPTX 35 kb)
Online Resource 3
(A) Chromatogram of the 10 μM internal standard (a). (B) Chromatogram of the 10 μM internal standard, 6.7 μM doxapram (b), and 7.3 μM keto-doxapram (c). (C) Chromatogram of LBW infants receiving a maintenance dose of 0.20 mg/kg/h after a loading dose of 1.5 mg/kg for 1 h. Values indicate the retention time. (PPTX 77 kb)
Online Resource 4
(A) Chromatogram of keto-doxapram (m/z 393). (B) Chromatogram of midazolam as the internal standard (m/z 326). (PPTX 72 kb)
Rights and permissions
About this article
Cite this article
Ogawa, Y., Irikura, M., Kobaru, Y. et al. Population pharmacokinetics of doxapram in low-birth-weight Japanese infants with apnea. Eur J Pediatr 174, 509–518 (2015). https://doi.org/10.1007/s00431-014-2416-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-014-2416-1