Abstract
The prevalence of obesity has exponentially risen worldwide. The etiology of obesity is multifactorial, and genetic inheritance and behavioral/environmental causes are considered the main etiological factors. Moreover, evidence that specific infections might promote the development of obesity has steadily accumulated. Only a few works investigate the impact of obesity on the immune response to infections and the risk of infections in the obese population. The aim of this paper was to review the available data regarding the various aspects of the association between obesity and infections and to highlight the possibility that infectious agents may have an etiological role in obesity, an idea known as “infectobesity”. Several microbes have been considered as possible promoter of obesity, but most of the data concern adenovirus-36 that exerts an adipogenic action mainly via a direct effect on adipose tissue leading to weight gain, at least in animal models.
Obesity affects the immune response leading to an increased susceptibility to infections. Obese adults and children show an increased incidence of both nosocomial and community-acquired infections. Furthermore, obesity may alter the pharmacokinetics of antimicrobial drugs and impact on vaccine response. However, the various aspects of the association of obesity infections remain poorly studied, and a call to research is necessary to better investigate the problem.
In conclusion, obesity impacts millions globally, and greater understanding of its etiology and its effects on immunity, infections, and prevention and management strategies is a key public health concern.
Similar content being viewed by others
Introduction
Overweight and obesity are a major public health concern both in adults and in children. In 1997, the World Health Organization has declared obesity a global epidemic [84], and the Healthy People 2010 has identified overweight and obesity an indicator of the health of a given population [30]. The prevalence of obesity in children and adolescents has increased over several decades in many industrialized countries although in the most recent years, it seems to have reached a plateau [60]. It affects about 17 % of children and adolescents aged 2–19 years in the US [59] and about 8 % and 12 % of school-age children in Europe and in Italy, respectively [10, 77]. The etiology of obesity is multifactorial, and genetic inheritance and behavioral/environmental causes are considered the most important factors leading to fat accumulation in children [49]. Twin studies have clearly demonstrated a genetic risk of obesity, and the discovery of leptin, ghrelin, adiponectin, and other hormones that influence appetite, satiety, and fat distribution provides insights into metabolic mechanisms for a physiologic risk. However, the exponential increase in the prevalence of childhood obesity in the past decades results from changes in eating and physical activity behaviors that have modified the balance between energy intake and energy expenditure although their influence is difficult to determine [5]. Moreover, in some cases, none of these factors is entirely sufficient to explain obesity [38]. An early identification of the obese population is crucial both to prevent and to detect comorbidities that compromise the quality of life and increase overall mortality [56] particularly as most cases remain obese into adult age [29]. Obese children are in fact a population at risk for developing metabolic, endocrine, gastroenterological, cardiovascular, respiratory, orthopedic, dermatological, neurological, and psychological complications. All of these complications, although not completely clarified, have been widely studied while few papers investigate the impact of obesity on the immune response to infections and the risk of infections in an overweight population. The aim of this review was to summarize the available evidence regarding the association between obesity and infections, including (1) obesity-related mechanisms that lead to predisposition to infections, (2) the epidemiology of nosocomial and community-acquired infections in the obese population, and the (3) management of infections in the obese adults and children. To do it, we searched PubMed, Cochrane, and EMBASE (January 1980–December 2012) to identify studies evaluating the various aspects of the association between obesity and infections. The search terms used included “obesity,” “infections” or “infectious diseases,” “immune system” or “immunity,” “children” (or “adolescents”), “adults,” “antimicrobial drugs” or “antibacterial drugs,” “vaccination” or “vaccine,” and “infectobesity”. These terms were combined in various ways to generate a wide search. In addition, we checked the references of eligible articles for further papers that were not captured by our search strategy and corresponded with authors when a full-length article was not available directly online or when relevant information was missing in the paper. We included only studies in English and both animal and human studies. We evaluated both children and adolescents studies and adults ones. Every type of study design was included.
Mechanisms that predispose obese patients to infection
Recent studies have demonstrated altered immune cell function in obese human subjects compared with those of healthy weight. Nieman et al. reported significant differences in leucocyte and lymphocyte subset counts and higher monocyte and granulocyte phagocytosis and oxidative burst activity in obese adult subjects [57]. Furthermore, obese patients show a proinflammatory state of circulating mononuclear cells compared with healthy-weight persons [27]. Additionally, obesity enhances thymic aging and reduces T cell repertoire diversity [85]. The reported findings suggest that obesity may result in an altered immune surveillance and in an impaired host defense. It is well known that obesity is associated with a state of chronic, low-grade inflammation both in white adipose tissue and at systemic level and that adipose tissue participates actively in inflammation and immunity [12, 22, 71]. Therefore, obesity is characterized by altered levels of circulating hormones and nutrients. Circulating immune cells and those resident in peripheral tissues are thus exposed to an energy-rich environment in the context of altered concentrations of metabolic factors and hormones [51]. The primary immunomodulatory adipokines include leptin, adiponectin, and the proinflammatory cytokines TNFα, IL-6, and IL-1β [22, 71]. Adiponectin, levels of which are decreased in obesity, is potently immunosuppressive [82]. In contrast, leptin plays a proinflammatory role. It induces polymorphonuclear neutrophils chemotaxis and reactive oxygen species generation [13, 52], up-regulates monocytes proinflammatory cytokine production and phagocytosis [42], and improves proliferation and cytotoxicity of natural killer cells [87]. Furthermore, leptin exerts an influence on the adaptive arm of the immune response, playing a key role in lymphopoiesis and myelopoiesis and representing a source of prosurvival signals to thymocytes during the maturation of T cells [14, 43]. Leptin induction seems to be a protective component of the immune response, and genetic leptin deficiency has been associated with increased mortality due to infections both in rodent models [48] and in humans [61]. The most used genetically altered obese animal models are the ob/ob and db/db mice lacking leptin and the leptin receptor, respectively. These mice show an increased susceptibility to bacterial and fungal infections although it is difficult to determine if the adiposity excess plays a direct or a secondary role on the immune responses [31, 32, 48, 65]. In humans, leptin-deficient children have normal weight at birth but then rapidly display hyperphagia and early-onset obesity in late infancy [23]. Along with obesity, the presence of immune dysfunction and the development of infections early in life are among the criteria to perform the genetic analysis. Farooqi et al. showed in three morbidly obese children, who were congenitally deficient in leptin, that the deficiency of the adipokine was associated with reduced numbers of circulating CD4+ T cells and impaired T cell proliferation and cytokine release, all of which were reversed by recombinant human leptin administration, opening new therapeutic perspectives and assuming new clinical applications [24]. However, these models do not completely fit non-genetically induced obesity, which constitutes the vast majority of human obesity. Moreover, many papers have considered the fact that obese individuals show hyperleptinemia and central and peripheral leptin resistance. Diet-induced obesity (DIO) mice more closely mimic human obesity. They show several forms of immunity impairment and a higher morbidity and mortality during viral infections although pathophysiological mechanisms have not yet been clarified [37, 68]. Even the use of DIO mice as experimental models presents critical issues as the greater susceptibility to infections can be related to the abundance of adipose tissue, the influence of the high-fat diet or both. Using both genetic obesity models and DIO mice could fill the gap in the knowledge of the impact of fat excess and diet on the immune response.
Obesity is also characterized by metabolic alterations, and hyperinsulinemia and insulin resistance are common features even in obese children [66]. Some cells involved in the immune response, such as monocytes [72], express the insulin receptor while in other cells, such as T cells, the expression of this receptor may be induced by a polyclonal stimulator or a specific antigen [75]. Insulin can modulate T cell activation and function, promoting an anti-inflammatory T helper type 2 cell phenotype [75]. On the other hand, insulin resistance has been related to a proinflammatory T helper type 1 cell response [47]. Although it is clear that insulin can exert several immunomodulatory effects, little is known about the consequences of excess insulin and insulin resistance on the immune system in the context of obesity. Glucose and fatty acids are important sources of energy for host defense and immune function, but elevated levels of these nutrients, as in the obese, have been demonstrated to alter the immune response. The exposition of T cells to high glucose concentrations causes the production of reactive oxygen species and lipid peroxidation [70] and, in mouse T-cells, overexpression of GLUT1 resulted in altered T cell metabolism and cytokine production [34]. Similarly, an excess of fatty acids may be a trigger both for innate and for adaptive immune response [78]. Obesity is thus characterized by complex metabolic and hormonal changes each of which interacts with the immune system; more studies are needed to better understand the consequences of obesity on the host response to infection.
Infections in clinical settings: studies in adults
Several studies reported that obesity increases susceptibility to infection in various clinical settings. Obese individuals show an increased incidence of both nosocomial and community-acquired infections. Most of the epidemiological data concern adult subjects. Obese patients show increased intensive care unit (ICU) length of stay and hospital mortality [8]. Obesity represents an independent risk factor for surgical site infections following various surgical procedures [76]. The increase of adipose tissue and of local tissue damage related to retraction, longer operative time, disturbance of body homeostatic balance, and other local changes may contribute to the increased incidence of surgical site infections. Moreover, obese patients show a relative hypoxia of subcutaneous tissue, and this may predispose to wound infections. Furthermore, obese subjects are more prone to experience prolonged hospitalizations due to a more difficult management, thus increasing their risk of acquiring a nosocomial infection [21].
Obesity can profoundly alter lung mechanics and enhances pulmonary inflammation which could potentially increase the risk for pneumonia or other lung infections [39]. Moreover, obesity is closely associated with obstructive sleep apnea syndrome (OSAS) that is associated with an increased risk for aspiration. In adult women, high body mass index (BMI) was shown to be associated with an increased risk of community-acquired pneumonia [21]. Several studies on the 2009 influenza A (H1N1) pandemic strain have demonstrated a worse outcome of infection in obese subjects that showed a greater number of hospitalizations, a longer duration of ICU admission and mechanical ventilation, and a higher rate of mortality compared with normal-weight patients [44]. Before the advent of the H1N1 pandemic, no study was published on the relationship between obesity and influenza infection in human subjects [51]. Kwong et al. demonstrated with a retrospective study that covered 12 influenza seasons that obese subjects present a greater risk for respiratory hospitalizations during the seasonal flu periods [41]. These data have a strong clinical impact, suggesting that obese individuals should be treated promptly and should undergo vaccination or antiviral therapy with priority during pandemics. Conversely, other studies have reported that obese individuals are not at greater risk for respiratory infections [17]. Therefore, our understanding of the effects of obesity on risk for pulmonary infection remains unclear, and more studies are needed.
Obesity increases risk of gastrointestinal complications such as non-alcoholic fatty liver disease (NAFLD). Several authors have demonstrated that obesity is a risk factor for the development of steatosis in patients with chronic hepatitis C infection, as BMI correlates with the grade of steatosis [86]. Steatosis can impact on the natural course of hepatitis C infection, being associated with fibrosis, reducing the response to antiviral treatment and contributing to the risk of hepatocellular carcinoma. Consequently, hepatitis C infection progresses more rapidly in obese compared with normal-weight patients.
Moreover, obese subjects are more prone to develop various skin infections such as intertrigo, candidiasis, furunculosis, erythrasma, tinea cruris, and folliculitis and to show rapidly progressive bacterial bone and joint infections [21].
Infections in clinical settings: pediatric studies
A few studies have investigated the risk of infections in obese children and adolescents. Indeed, with the regard to surgical site infections, children who undergo complicated surgeries such as children with congenital heart diseases, generally show a normal or a low weight. More data can be found about the incidence of respiratory tract infections in obese children. Even in childhood, obesity is associated with profound alterations of respiratory mechanics and physiology. Obesity represents one of the four clinical phenotypes associated with OSAS in children. The excess of body fat can lead to a reduction in upper airways caliber due to the mechanical effect on soft tissues, increased the airway collapsibility, reduced lung compliance, and increased respiratory work. Moreover, in obese children, higher body weight is associated with a decrease in nocturnal oxygen saturation [40], and they show an altered central response to hypoxia and hypercapnia [2]. Furthermore, leptin resistance seems to be associated to hypercapnia, and ob/ob mice show a suppressed respiratory response to this stimulus [33, 67]. OSAS and obesity, via neuroendocrine changes and oxidative stress, enhance inflammation and cytokine production and reduce nitric oxide production [9]. All these mechanisms may alter the response of the host to infectious agents causing an increased susceptibility of obese children to serious respiratory infections. Jedrychowski et al. have analyzed factors predisposing to recurrent acute respiratory infections in 1,129 9-year-old school children. Susceptibility to acute respiratory infections was significantly associated with BMI; overweight children (BMI ≥ 20 kg/m2) had twice the risk of infections compared with children with a lower BMI (OR 2 · 02, 95 % CI 1 · 13–3 · 59) [35]. Akiyama et al. showed in 243 children admitted for bronchitis, bronchiolitis, pneumonia, and those who were positive for a respiratory syncytial virus (RSV) infection that days of wheezing, days of fever, and days of drip infusion were positively associated with obesity ratio [1]. However, only these studies are available on a significant wide cohort of pediatric patients, and more data are needed to better understand the problem.
Obese subjects show the presence of specific bacteria in the oral flora since obesity is often related to increased sugar consumption and other comorbidities such as diabetes mellitus. This leads to the production of proinflammatory cytokines and reactive oxygen species and to oral inflammation and oxidative stress. It has been reported that obesity is a predisposing factor for periodontal disease, especially among children and adolescents [83]. Willershausen et al. showed in 842 elementary school children that 35.5 % of the pupils with normal weight had healthy teeth, whereas the number dropped to 27.5 % in children that were overweight and to 29.7 % in obese children. The caries prevalence also showed a significant association with weight [81]. Furthermore, Franchini et al. demonstrated that overweight/obese children (age 10–17 years) had a worse attitude towards oral hygiene. In fact, they revealed an effect of obesity status on the gingival index and gingivitis that was dependent on insulin resistance and poor oral hygiene rather than simply on the overweight/obese status. Authors also showed that in obese children, negative psychological features were also risk factors for gingivitis probably because they were related to a generic low self-esteem [25].
Moreover, obese children are at risk for developing severe skin infections as Staphylococcus aureus infections [18], and obesity is associated with dermatoses as skin tags, striae distensae, and plantar hyperkeratosis and with an altered epidermal permeability barrier status that can predispose to cutaneous infections [58].
In conclusion, the studies on the association between obesity and infection in children show that obese pediatric subjects are a population at risk for respiratory, odontogenic, and cutaneous infections. However, more studies are needed to explore this topic.
Management of infections and vaccination in obese patients
Despite the growing prevalence of obesity worldwide and the increased risk for several types of microbial infections, there are no well-defined guidelines about the management of infections in obese patients and a little is known about how obesity may alter the pharmacokinetics of antimicrobial drugs increasing distribution volume and alter the clearance. Studies assessing dosing of antibacterial drugs including vancomycin, aminoglycosides, cephalosporins, and linezolid showed that obese subjects may require different dosages and different dosing intervals compared with non-obese individuals [6, 7, 69]. Moreover, all these studies are performed in adult cohorts while there is no study on pediatric age also because the drugs doses are usually “per kilogram of body weight”.
Furthermore, there is some evidence that obese subjects may respond to vaccination less than normal-weight individuals. Obesity was associated with a poor antibody response to hepatitis B vaccination [79]. Eliakim et al. showed that anti-tetanus IgG antibodies were significantly lower in the overweight children compared to that of the normal weight controls. This reduced response to vaccinations in obese children and adolescent might be due to mechanical factors such as a lower relative vaccination dose, or reduced absorption from the injection site due to increased adipose tissue, or related to reduce immune response due to the chronic low-grade inflammation [19]. Interestingly, a recent study reported that using longer vaccine needles resulted in a higher antibody titers to hepatitis B surface antigen in obese adolescents [50]. Poor responses to vaccination and to antimicrobial drugs have important public health implications, and a careful consideration of infection prevention and treatment in obese subjects is required in the future.
Infectobesity
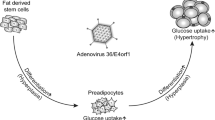
Evidence that specific infections might promote the development of obesity has steadily accumulated over the past 25 years [46]. Infectobesity, or obesity of infectious origin, creates new perspectives for prevention strategies and treatment options as pathogen-specific vaccines, the restriction of the spread of infection and antimicrobial therapies. Several microbes have been considered as possible promoter of obesity, but most of the data have been collected in studies concerning adenoviruses [20]. Human adenoviruses are a group of icosahedral, non-enveloped, double-stranded DNA viruses categorized into seven subgroups A to G according to their antigens. Human adenoviruses are implicated in infections of the upper respiratory and gastrointestinal tracts and in conjunctivitis [45]. Most adenovirus infections are acquired in childhood. Cidofovir is considered the drug of choice for the treatment of severe infections, but new drugs are under investigation. Moreover, vaccines effective in preventing AD-4 and AD-7 infections have been developed [45]. AD-36, a human adenovirus belonging to subgroup D, was the first human adipogenic virus reported. The virus was first isolated in the feces of a girl with enteritis and that suggests fecal–oral route of transmission [80]. The most important mechanism of the adipogenic action of AD-36 is a direct effect on adipose tissue [63]. In vivo and in vitro studies demonstrated that AD-36 upregulates the proliferation, adipogenic commitment, and differentiation of adult adipose tissue-derived stem cells and other adipogenic progenitors, leading to an increase in the number of fat cells [64]. Moreover, AD-36 infection seems to determine the early appearance of enzymes involved in lipid and glucose uptake, the accumulation of triglycerides and a reduction of leptin expression and secretion leading to an increase in appetite and a further increase in fat tissue [74]. Furthermore, AD-36 supports chronic inflammation inducing the macrophages infiltration into adipocytes [55]. Pasarica et al. demonstrated that AD-36 infection can determine alterations on the central nervous system (CNS), changing norepinephrine levels in the paraventricular nucleus and decreasing corticosterone secretion which plays a major role in fat metabolism [62]. A series of studies demonstrated that infection with AD-36 increases adiposity in various animal models [16, 36, 62, 77]. AD-36 infection significantly increases body weight, reduces serum total cholesterol and triglyceride concentrations, and determines a shift from high-density lipoprotein to low-density lipoprotein cholesterol [15, 36]. Dhurandhar et al. also showed the possibility of horizontal transmission of the infection. Additionally, they demonstrated that the transfusion of a small amount of blood from AD-36-infected animals to uninfected ones successfully transmitted infection, and the recipients subsequently became obese [16].
Human studies on the association between obesity and AD-36 infection reported discordant results (Table 1). The greatest differences can be found in adult data as some studies indicate a close relationship between serum AD-36 antibodies and BMI or weight gain while others do not confirm this association [3, 11, 28, 54, 73]. The strongest evidence of the adipogenic role of AD-36 in humans comes from a study that screened 90 sets of twins, 26 of them were discordant for AD-36 infection seropositivity. Atkinson et al. demonstrated that the AD-36-seropositive twin had a significantly higher BMI and more body fat than the seronegative sibling [3]. Similarly, discordant results have been reported on the effects of AD-36 infections on total cholesterol and triglyceride levels [3, 11, 28, 54, 73]. In comparison with adult studies, data regarding children are more consistent in establishing a positive correlation between obesity and AD-36 [4, 26, 53]. As Esposito et al. recently pointed out, various factors could explain these discordant results as the use of inappropriate methods of evaluating serum AD-36 antibodies and AD-36 DNA in fat tissue, the inclusion of some confounding factors as the time of infection, the viral load and the persistence of the virus in the body, the use of specific populations, and the age of the infected host [20].
The available data do not completely solve the problem of the possible role of AD-36 as a causative factor of obesity although it seems to be important at least in children. More well-designed longitudinal studies are needed to establish this association with hypotheses of important implications in terms of prevention and therapeutic options.
Conclusions
The best solution to improving health of obese individuals is the weight loss. However, the etiology of this complex disease is multifactorial, and a better definition of the various etiological factors would be essential to develop prevention and treatment strategies. The available data on the possible relationship between AD-36 infection and obesity do not completely solve the problem although AD-36 may be a contributing factor at least in some subjects, such as children.
Although obesity is a well-known risk factor for several morbid conditions, its relation to infections has not been adequately studied. The available evidence suggests that excess adiposity negatively impacts on immune function and host defense in obese individuals. Epidemiological data demonstrate that obese adults and children show an increased incidence of both nosocomial and community-acquired infections. Furthermore, rodent models offer important insights into how metabolic abnormalities associated with excess body weight can impair immunity. However, a call to research is necessary to investigate the specific aspects of immunity impaired in obesity and to better define the risk of specific type of infections.
Additionally, there are no well-defined guidelines about the management of infections in obese patients although some studies have shown that the excess of body fat may alter the pharmacokinetics of antimicrobial drugs. Furthermore, some findings show that obese subjects may respond to vaccination less than normal-weight individuals.
Obesity impacts millions globally, and greater understanding of its effects on immunity, infections, prevention and management strategies is a key public health concern that could likely save millions of lives.
References
Akiyama N, Segawa T, Ida H, Mezawa H, Noya M, Tamez S, Urashima M (2011) Bimodal effects of obesity ratio on disease duration of respiratory syncytial virus infection in children. Allergol Int 60:305–308
Arens R, Muzumdar H (2008) Childhood obesity and obstructive sleep apnea syndrome. J Appl Physiol 108:436–444
Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, Augustus AS (2005) Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes 29:281–286
Atkinson RL, Lee I, Shin HJ, He J (2010) Human adenovirus-36 antibody status is associated with obesity in chidren. Int J Pediatr Obes 5:157–160
Barlow SE, Committee E (2007) Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity. Summary Report. Pediatrics 120(Suppl 4):164–192
Bauer LA, Black DJ, Lill JS (1998) Vancomycin dosing in morbidly obese patients. Eur J Clin Pharmacol 54:621–625
Bearden DT, Rodvold KA (2000) Dosage adjustments for antibacterials in obese patients: applying clinical pharmacokinetics. Clin Pharmacokinet 38:415–426
Bercault N, Boulain T, Kuteifan K, Wolf M, Runge I, Fleury JC (2004) Obesity-related excess mortality rate in an adult intensive care unit: a risk-adjusted matched cohort study. Crit Care Med 32:998–1003
Bhattacharjee R, Kim J, Kheirandish-Gozal L, Gozal D (2011) Obesity and obstructive sleep apnea syndrome in children: a tale of inflammatory cascades. Pediatr Pulmonol 46:313–323
Binkin N, Fontana G, Lamberti A, Cattaneo C, Baglio G, Perra A, Spinelli A (2010) A national survey of the prevalence of childhood overweight and obesity in Italy. Obes Rev 11:2–10
Broderick MP, Hansen CJ, Irvine M, Metzgar D, Campbell K, Baker C, Russell KL (2010) Adenovirus 36 seropositivity is strongly associated with race and gender, but not obesity, among US military personnel. Int J Obes 34:302–308
Bulló M, García-Lorda P, Megias I, Salas-Salvadó J (2003) Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obesity 11:525–531
Caldefie-Chezet F, Poulin A, Vasson MP (2003) Leptin regulates functional capacities of polymorphonuclear neutrophils. Free Radic Res 37:809–814
Claycombe K, King LE, Fraker PJ (2008) A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci U S A 105:2017–2021
Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL (2000) Increased adiposity in animals due to a human virus. Int J Obes Relat Metab Disord 24:989–996
Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL (2001) Transmissibility of adenovirus-induced adiposity in a chicken model. Int J Obes Relat Metab Disord 25:990–996
Dossett LA, Heffernan D, Lightfoot M, Collier B, Diaz JJ, Sawyer RG, May AK (2008) Obesity and pulmonary complications in critically injured adults. Chest 134:974–980
Early GJ, Seifried SE (2012) Risk factors for community-associated Staphylococcus aureus skin infection in children of Maui, Hawaii. J Med Public Health 71:218–223
Eliakim A, Schwindt C, Zaldivar F, Casali P, Cooper DM (2006) Reduced tetanus antibody titers in overweight children. Autoimmunity 39:137–141
Esposito S, Preti V, Consolo S, Nazzari E, Principi N (2012) Adenovirus 36 infection and obesity. J Clin Virol 55:95–100
Falagas ME, Kompoti M (2006) Obesity and infection. Lancet Infect Dis 6:438–446
Fantuzzi G (2005) Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 115:911–919
Farooqi IS (2008) Monogenic human obesity. Front Horm Res 36:1–11
Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S (2002) Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110:1093–1094
Franchini R, Petri A, Migliario M, Rimondini L (2011) Poor oral hygiene and gingivitis are associated with obesity and overweight status in paediatric subjects. J Clin Periodontol 38:1021–1028
Gabbert C, Donohue M, Arnold J, Schwimmer JB (2010) Adenovirus 36 and obesity in children and adolescents. Pediatrics 126:721–726
Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P (2004) Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 110:1564–1571
Goossens VJ, deJager SA, Grauls GE, Gielen M, Vlietinck RF, Derom CA, Loos RJ, Rensen SS, Buurman WA, Greve JW, van Baak MA, Wolffs PF, Bruggeman CA, Hoebe CJ (2011) Lack of evidence for the role of human adenovirus-36 in obesity in a European cohort. Obesity 19:220–221
Guo SS, Chumlea WC (1999) Tracking of body mass index in children in relation to overweight in adulthood. Am J Clin Nutr 70(1 Part 2):145–148
Healthy People (2010) DC: US Dept of Health and Human Services, 2nd ed. Washington 2000
Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P (2007) Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol 150:332–339
Ikejima S, Sasaki S, Sashinami H, Mori F, Ogawa Y, Nakamura T, Abe Y, Wakabayashi K, Suda T, Nakane A (2005) Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes 54:182–189
Inyushkina EM, Merkulova NA, Inyushkin AN (2010) Mechanisms of the respiratory activity of leptin at the level of the solitary tract nucleus. Neurosci Behav Physiol 40:707–713
Jacobs SR, Herman CE, MacIver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC (2008) Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol 180:4476–4486
Jedrychowski W, Maugeri U, Flak E, Mroz E, Bianchi I (1998) Predisposition to acute respiratory infections among overweight preadolescent children: an epidemiologic study in Poland. Public Health 112:189–195
Kapila M, Khosla P, Dhurandhar NV (2004) Novel short-term effects of adenovirus AD-36, on hamster lipoproteins. Int J Obes Relat Metab Disord 28:1521–1527
Karlsson EA, Sheridan PA, Beck MA (2010) Dietinduced obesity impairs the T cell memory response to influenza virus infection. J Immunol 184:3127–3133
Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, Ruden D, Pietrobelli A, Barger JL, Fontaine KR, Wang C, Aronne LJ, Wright SM, Baskin M, Dhurandhar NV, Lijoi MC, Grilo CM, DeLuca M, Westfall AO, Allison DB (2006) Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes 30:1585–1594
Koenig SM (2001) Pulmonary complications of obesity. Am J Med Sci 321:249–279
Korndewal MJ, Geurts van Kessel WM, Jak LG, Uiterwaal CS, Rövekamp MH, van der Ent CK (2012) Influence of obesity on nocturnal oxygen saturation in young children. Eur J Pediatr 171:1687–1693
Kwong JC, Campitelli MA, Rosella LC (2011) Obesity and respiratory hospitalizations during influenza seasons in Ontario, Canada: a cohort study. Clin Infect Dis 53:413–421
Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM (1998) Leptin regulates proinflammatory immune responses. FASEB J 2:57–65
Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI (1998) Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897–901
Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, Matyas BT, California Pandemic (H1N1) Working Group (2011) A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 52:301–312
Lynch JP 3rd, Fishbein M, Echavarria M (2011) Adenovirus. Semin Respir Crit Care Med 32:494–511
Lyons MJ, Faust IM, Hemmes RB, Buskirk DR, Hirsch J, Zabriskie JB (1982) A virally induced obesity syndrome in mice. Science 216:82–85
Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC (2008) Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol 84:949–957
Mancuso P (2010) Obesity and lung inflammation. J Appl Physiol 108:722–728
McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, Benca RM, Biggio J, Boggiano MM, Eisenmann JC, Elobeid M, Fontaine KR, Gluckman P, Hanlon EC, Katzmarzyk P, Pietrobelli A, Redden DT, Ruden DM, Wang C, Waterland RA, Wright SM, Allison DB (2009) Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 49:868–913
Middleman AB, Anding R, Tung C (2010) Effect of needle length when immunizing obese adolescents with hepatitis B vaccine. Pediatrics 125:508–512
Milner JJ, Beck MA (2012) The impact of obesity on the immune response to infection. Proc Nutr Soc 71:298–306
Montecucco F, Bianchi G, Gnerre P, Bertolotto M, Dallegri F, Ottonello L (2006) Induction of neutrophil chemotaxis by leptin: crucial role for p38 and Src kinases. Ann N Y Acad Sci 1069:463–471
Na HN, Hong YM, Kim J, Kim HN, Jo I, Nam JH (2010) Association between human adenovirus-36 and lipid disorders in Korean schoolchildren. Int J Obes 34:89–93
Na HN, Kim J, Lee HS, Shim KW, Kimm H, Jee SH (2012) Association of human adenovirus-36 in overweight Korean adults. Int J Obes 36:281–285
Na HN, Nam JH (2012) Adenovirus 36 as an obesity agent maintains the obesity state by increasing MCP-1 and inducing inflammation. J Infect Dis 205:914–922
Nadeau KJ, Maahs DM, Daniels SR, Eckel RH (2011) Childhood obesity and cardiovascular disease: links and prevention strategies. Nat Rev Cardiol 8:513–525
Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, Butterworth DE, Fagoaga OR (1999) Influence of obesity on immune function. J Am Diet Assoc 99:294–299
Nino M, Franzese A, Ruggiero Perrino N, Balato N (2012) The effect of obesity on skin disease and epidermal permeability barrier status in children. Pediatr Dermatol 29:567–570
Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief 82:1–8
Olds T, Maher C, Zumin S, Péneau S, Lioret S, Castetbon K, Bellisle, de Wilde J, Hohepa M, Maddison R, Lissner L, Sjöberg A, Zimmermann M, Aeberli I, Ogden C, Flegal K, Summerbell C (2011) Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. Int J Pediatr Obes 6:342–360
Ozata M, Ozdemir LC, Licinio J (1999) Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin of leptin mediated defects. J Clin Endocrinol Metab 84:3686–3695
Parsarica M, Shin AC, Yu M, Ou Yang HM, Rathod M, Jen KL, MohanKumar S, MohanKumar PS, Markward N, Dhurandhar NV (2006) Human adenovirus 36 induces adiposity, increases insulin sensitivity, and alters hypothalamic monoamines in rats. Obesity 14:1905–1913
Pasarica M, Dhurandhar NV (2007) Infectobesity: obesity of infectious origin. Adv Food Nutr Res 52:61–102
Pasarica M, Mashtalir N, McAllister EJ, Kilroy GE, Koska J, Permana P, de Courten B, Yu M, Ravussin E, Gimble JM, Dhurandhar NV (2008) Adipogenic human adenovirus Ad-36 induces commitment, differentiation and lipid accumulation in human adipose-derived stem cells. Stem Cells 26:969–978
Plotkin BJ, Paulson D, Chelich A, Jurak D, Cole J, Kasimos J, Burdick JR, Casteel N (1996) Immune responsiveness in a rat model for type II diabetes (Zucker rat, fa/fa): susceptibility to Candida albicans infection and leucocyte function. J Med Microbiol 44:277–283
Prodam F, Ricotti R, Genoni G, Parlamento S, Petri A, Balossini C, Savastio S, Bona G, Bellone S (2013) Comparison of two classifications of metabolic syndrome in the pediatric population and the impact of cholesterol. J Endocrinol Invest 36:466–473
Rabec C, de Lucas RP, Veale D (2011) Respiratory complications of obesity. Arch Bronconeumol 47:252–261
Smith AG, Sheridan PA, Tseng RJ, Sheridan JF, Beck MA (2009) Selective impairment in dendritic cell function and altered antigenspecific CD8 T-cell responses in diet-induced obese mice infected with influenza virus. Immunology 26:268–279
Stein GE, Schooley SL, Peloquin CA, Kak V, Havlichek DH, Citron DM, Tyrrell KL, Goldstein EJ (2005) Pharmacokinetics and pharmacodynamics of linezolid in obese patients with cellulitis. Ann Pharmacother 39:427–432
Stentz FB, Kitabchi AE (2005) Hyperglycemia-induced activation of human T-lymphocytes with de novo emergence of insulin receptors and generation of reactive oxygen species. Biochem Biophys Res Commun 335:491–495
Tilg H, Moschen AR (2006) Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6:772–783
Trischitta V, Brunetti A, Chiavetta A, Benzi L, Papa V, Vigneri R (1989) Defects in insulin-receptor internalization and processing in monocytes of obese subjects and obese NIDDM patients. Diabetes 38:1579–1584
Trovato GM, Castro A, Tonzuso A, Garozzo A, Martines GF, Pirri C, Trovato F, Catalano D (2009) Human obesity relationship with Ad36 adenovirus and insulin resistance. Int J Obes 33:1402–1409
Vangipuram SD, Yu M, Tian J, Stanhope KL, Pasarica M, Havel PJ, Heydari AR, Dhurandhar NV (2007) Adipogenic human adenovirus-36 reduces leptin expression and secretion and increases glucose uptake by fat cells. Int J Obes 31:87–96
Viardot A, Grey ST, Mackay F, Chisholm D (2006) Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology 148:346–353
Vilar-Compte D, Mohar A, Sandoval S, de la Rosa M, Gordillo P, Volkow P (2000) Surgical site infections at the National Cancer Institute in Mexico: a case–control study. Am J Infect Control 28:14–20
Wang Y, Lobstein T (2006) Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 1:11–25
Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH (2005) Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol 174:5390–5397
Weber DJ, Rutala WA, Samsa GP, Bradshaw SE, Lemon SM (1986) Impaired immunogenicity of hepatitis B vaccine in obese persons. N Engl J Med 314:1393–1393
Wigand R, Gelderblom H, Wadell G (1980) New human adenovirus (candidate adenovirus 36), a novel member of subgroup D. Arch Virol 64:225–233
Willershausen B, Haas G, Krummenauer F, Hohenfellner K (2004) Relationship between high weight and caries frequency in German elementary school children. Eur J Med Res 9:400–404
Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H (2004) Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 323:630–635
Wood N, Johnson RB, Streckfus CF (2003) Comparison of body composition and periodontal disease using nutritional assessment techniques: Third National Health and Nutrition Examination Survey (NHANES III). J Clin Periodontol 30:321–327
World Health Organization (2001) Obesity: preventing and managing the global epidemic. Report of a WHO Consultation on Obesity, 3–5 June 1997, Geneva 2001. WHO/NUT/NCD 98.1
Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, Dixit VD (2009) Obesity accelerates thymic aging. Blood 114:3803–3812
Younossi ZM, McCullough AJ, Ong JP, Barnes DS, Post A, Tavill A, Bringman D, Martin LM, Assmann J, Gramlich T, Mullen KD, O'Shea R, Carey WD, Ferguson R (2004) Obesity and non-alcoholic fatty liver disease in chronic hepatitis C. J Clin Gastroenterol 38:705–709
Zhao Y, Sun R, You L, Gao C, Tian Z (2003) Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun 300:247–252
Conflict of interests
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Genoni, G., Prodam, F., Marolda, A. et al. Obesity and infection: two sides of one coin. Eur J Pediatr 173, 25–32 (2014). https://doi.org/10.1007/s00431-013-2178-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-013-2178-1