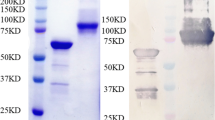
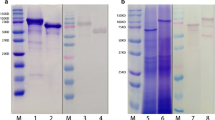
Abstract
Streptococcuspneumoniae, or pneumococcus, is a major respiratory-tract pathogen that causes high levels of mortality and morbidity in infants and elderly individuals. Despite the development of various capsular polysaccharide vaccines to prevent pneumococcal disease, it remains epidemic. Pneumococcal surface protein A (PspA) is a highly immunogenic surface protein existing in all strains of S. pneumoniae, and it can elicit immunizing protection against pneumococcal infection. In our previous studies, a fusion protein (PsaA-PspA23), consisting of PspA and pneumococcal surface antigen A (PsaA), displayed greater immunogenicity and provided better protection in mice against S. pneumoniae strains than either PsaA or PspA. In this study, the fusion protein PsaA-PspA23, together with PspA4, was formulated with four adjuvants Al(OH)3, MF59, AS03, and AS02, and subsequently subjected to dose optimization and immunological evaluation for determination of the antibody titers, bacterial burden, survival rates, and levels of cytokines in mice. All vaccines with high adjuvant doses displayed higher antigen-specific immunoglobulin G (IgG) titers. Bacterial burdens were notably decreased to different extents in the lungs and blood of mice immunized with the antigen and various adjuvants. Among these adjuvants, AS02 provided outstanding protection against challenge with pathogenic bacteria from different families and clades; it also induced high titers of IgG1 and IgG2a. Moreover, only AS02 elicited high levels of cytokines, such as TNF-α, IFN-γ, IL-2, and IL-4. These results suggest that PsaA-PspA23 and PspA4 formulated with AS02 may potentially be used as a subunit vaccine against deadly pneumococcal infection.






Similar content being viewed by others
References
Pletz MW, Maus U, Krug N, Welte T, Lode H (2008) Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents 32:199–206
Pichichero ME (2017) Pneumococcal whole-cell and protein-based vaccines: changing the paradigm. Expert Rev Vac 16:1181–1190
Zhanel GG, James KD, Karlowsky WA (2015) Clinical cure rates in subjects treated with azithromycin for community-acquired respiratory tract infections caused by azithromycin-susceptible or azithromycin-resistant Streptococcus pneumoniae. J Antimicrob Chemother 3170
Bryce J, Boschi-Pinto C, Shibuya K, Black RE (2005) WHO estimates of the causes of death in children. Lancet 365:1147–1152
Lin H, Peng Y, Lin Z, Zhang S, Guo Y (2015) Development of a conjugate vaccine against invasive pneumococcal disease based on capsular polysaccharides coupled with PspA/family 1 protein of Streptococcus pneumoniae. Microb Pathogen 83–84:35–40
Shinefild RH, Steve Black M (2000) Efficacy of pneumococcal conjugate vaccines in largescale field trials. Pediatr Infect Dis J 19:394–397
Hsu HE et al (2009) Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. The New Engl J Med 360:244–256
Janulczyk R, Iannelli F, Sjoholm AG, Pozzi G, Bjorck L (2000) Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J Biol Chem 275:37257–37263
Dave S, Brooks-Walter A, Pangburn MK, McDaniel LS (2001) PspC, a pneumococcal surface protein, binds human factor H. Infect Immun 69:3435–3437
McKay JT et al (2015) PD-1 suppresses protective immunity to Streptococcus pneumoniae through a B cell-intrinsic mechanism. J Immunol 194:2289–2299
Crain MJ et al. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. 58, 3293–3299 (1990)
Tu AH, Fulgham RL, McCrory MA, Briles DE, Szalai AJ. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immunol 67:4720–4724 (1999)
Croney CM, Coats MT, Nahm MH, Briles DE, Crain MJ (2012) PspA family distribution, unlike capsular serotype, remains unaltered following introduction of the heptavalent pneumococcal conjugate vaccine. Clin Vac Immunol: CVI 19:891–896
Ren B, Szalai AJ, Hollingshead SK, Briles DE (2003) Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect Immun 72:114–122
Moreno AT et al (2010) Immunization of mice with single PspA fragments induces antibodies capable of mediating complement deposition on different pneumococcal strains and cross-protection. Clin Vac Immunol 17:439–446
Briles DE et al (2000) Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis 182:1694–1701
Goulart C et al (2011) Selection of family 1 PspA molecules capable of inducing broad-ranging cross-reactivity by complement deposition and opsonophagocytosis by murine peritoneal cells. Vaccine 29:1634
Whaley MJ et al (2010) Concomitant administration of recombinant PsaA and PCV7 reduces Streptococcus pneumoniae serotype 19A colonization in a murine model. Vaccine 28:3071
Oliveira MLS et al (2006) Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infection 8:1016–1024
Lu J et al (2015) Protective immune responses elicited by fusion protein containing PsaA and PspA fragments. Immunological investigations 44:482
Reed SG, Orr MT, Fox CB (2013) Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608
Shi L, Zhang YJ, Xue rui YI, Liu SR Cytotoxic T cell responses to hepatitis B virus (HBV) small surface antigen in mice. Chinese Journal of Nosoconmiology (2003)
Fang JH, Hora M (2000) The adjuvant MF59: a 10-year perspective gary ott. Ramachandran Radhakrishnan 42:211–228
Traquina P, Morandi M, Contorni M, Nest GV (1996) MF59 adjuvant enhances the antibody response to recombinant hepatitis B surface antigen vaccine in primates. J Infect Dis 174:1168–1175
Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A (2008) The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol 180:5402–5412
Calabro S et al (2011) Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29:1812–1823
Morel S et al (2011) Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 29:2461–2473
McElhaney JE et al (2013) AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis 13:485–496
Yam KK et al (2016) Comparison of AS03 and Alum on immune responses elicited by A/H3N2 split influenza vaccine in young, mature and aged BALB/c mice. Vaccine 34:1444–1451
Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P (2010) AS03(A)-adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis 51:668–677
Moris P et al (2011) H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol 31:443–454
Rénia L et al (2009) A Randomized Trial Assessing the Safety and Immunogenicity of AS01 and AS02 Adjuvanted RTS,S Malaria Vaccine Candidates in Children in Gabon. PloS one 4:e7611
Casimiro DR et al (2010) Efficacy of multivalent adenovirus-based vaccine against simian immunodeficiency virus challenge. J Virol 84:2996–3003
Beall B, Gherardi G, Facklam RR, Hollingshead SK (2000) Pneumococcal pspA sequence types of prevalent multiresistant pneumococcal strains in the United States and of internationally disseminated clones. J Clin Microbiol 38:3663–3669
Brandileone MC et al (2004) Typing of pneumococcal surface protein A (PspA) in Streptococcus pneumoniae isolated during epidemiological surveillance in Brazil: towards novel pneumococcal protein vaccines. Vaccine 22:3890–3896
Hollingshead SK et al (2006) Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J Med Microbiol 55:215–221
Echenique J, Kadioglu A, Romao S, Andrew PW, Trombe MC (2004) Protein serine/threonine kinase StkP positively controls virulence and competence in Streptococcus pneumoniae. Infect Immun 72:2434–2437
Miyaji EN et al (2015) Evaluation of a vaccine formulation against Streptococcus pneumoniae based on choline-binding proteins. Clin Vac Immunol: CVI 22:213–220
María Claudia Vela Coral et al (2001) Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates from colombian children. Emerg Infect Dis 7:5
Ogunniyi AD et al (2007) Contributions of pneumolysin, pneumococcal surface protein A (PspA), and PspC to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect Immun 75:1843–1851
Haughney SL et al (2013) Retention of structure, antigenicity, and biological function of pneumococcal surface protein A (PspA) released from polyanhydride nanoparticles. Acta Biomaterialia 9:8262–8271
Nabors GS et al (2000) Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743
Oliveira MLS et al (2010) Combination of pneumococcal surface protein A (PspA) with whole cell pertussis vaccine increases protection against pneumococcal challenge in mice. PloS one 5:e10863
Leroux-Roels I et al (2015) Adjuvant system AS02V enhances humoral and cellular immune responses to pneumococcal protein PhtD vaccine in healthy young and older adults: randomised, controlled trials. Vaccine 33:577–584
Piao Z et al (2014) Protective properties of a fusion pneumococcal surface protein A (PspA) vaccine against pneumococcal challenge by five different PspA clades in mice. Vaccine 32:5607–5613
Gordon CL et al (2012) Comparison of immunoglobulin G subclass concentrations in severe community-acquired pneumonia and severe pandemic 2009 influenza A (H1N1) infection. Clin Vac Immunol 19:446–448
Ekdahl K, Braconier JH, Svanborg C (1997) Immunoglobulin deficiencies and impaired immune response to polysaccharide antigens in adult patients with recurrent community-acquired pneumonia. Scand J Infect Dis 29:401–407
Acknowledgements
We are grateful for support from Jilin Provincial Industrial Innovation Special Project for the New Vaccine Adjuvant Innovation Technology Platform (Grant No. 2018C004).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical standards
All mouse experiments in this paper were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council).
Additional information
Edited by: B. Opitz.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Li, B., Yu, J. et al. Comparison of four adjuvants revealed the strongest protection against lethal pneumococcal challenge following immunization with PsaA-PspA fusion protein and AS02 as adjuvant. Med Microbiol Immunol 208, 215–226 (2019). https://doi.org/10.1007/s00430-019-00579-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-019-00579-9