Abstract
In order to investigate the involvement of sulfated groups in the Trypanosoma cruzi host–parasite relationship, we studied the interaction between the major cysteine proteinase of T. cruzi, cruzipain (Cz), a sulfate-containing sialylated molecule and the sialic acid-binding immunoglobulin like lectin-E (Siglec-E). To this aim, ELISA, indirect immunofluorescence assays and flow cytometry, using mouse Siglec-E–Fc fusion molecules and glycoproteins of parasites, were performed. Competition assays verified that the lectins, Maackia amurensis II (Mal II) and Siglec-E–Fc, compete for the same binding sites. Taking into account that Mal II binding remains unaltered by sulfation, we established this lectin as sialylation degree control. Proteins of an enriched microsomal fraction showed the highest binding to Siglec-E as compared with those from the other parasite subcellular fractions. ELISA assays and the affinity purification of Cz by a Siglec-E column confirmed the interaction between both molecules. The significant decrease in binding of Siglec-E–Fc to Cz and to its C-terminal domain (C-T) after desulfation of these molecules suggests that sulfates contribute to the interaction between Siglec-E–Fc and these glycoproteins. Competitive ELISA assays confirmed the involvement of sulfated epitopes in the affinity between Siglec-E and Cz, probably modified by natural protein environment. Interestingly, data from flow cytometry of untreated and chlorate-treated parasites suggested that sulfates are not primary receptors, but enhance the binding of Siglec-E to trypomastigotic forms. Altogether, our findings support the notion that sulfate-containing sialylated glycoproteins interact with Siglec-E, an ortholog protein of human Siglec-9, and might modulate the immune response of the host, favoring parasitemia and persistence of the parasite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, represents an important health problem in Latin America. The World Health Organization estimates that 8–15 million people currently are T. cruzi-infected in 18 endemic countries in Central and South America and approximately 50 million people are at risk [1]. The disease has also emerged as a public health problem elsewhere in the world due to infected people migrating to other regions [2, 3].
Cruzipain (Cz), the major cysteine proteinase (CP) of T. cruzi, bears in addition to a catalytic domain a carboxy-terminal extension (C-T), which is retained in the mature protein. The C-T domain is responsible for the immunodominant antigenic character of the molecule in natural and experimental infections [4–7]. Natural Cz is expressed as a complex mixture of isoforms by the different developmental stages of the parasite and presents microheterogeneities [8]. In addition, this lysosomal enzyme is a glycoprotein containing about 10 % carbohydrates [9] and three potential N-glycosylation sites, one of them in the C-T, carrying high-mannose, hybrid monoantennary or complex biantennary oligosaccharide chains [10, 11]. Moreover, it is a relevant virulence factor [12–14], which has been extensively studied as protease, glycoprotein and antigen [15], evaluated as candidate for vaccine development [16] and considered a very promising target for chemotherapy of the disease [17, 18]. In the last years, we have demonstrated the presence of a complex N-glycosidic oligosaccharide bearing sialic acid in Cz [19] and sulfated high-mannose-type oligosaccharides on the unique N-glycosylation site of the C-T domain [11]. Furthermore, we reported that these sulfate moieties are targets for specific immune responses [20] and that subjects chronically infected with T. cruzi mount specific humoral immune responses to this sulfated glycoprotein. Interestingly, our results showed that IgG2 antibody responses to sulfate groups on Cz and sulfatides inversely correlate with human severe chronic Chagas disease [20, 21].
Siglecs are a family of sialic acid-binding lectins, mainly expressed on cells of the immune system, which have in their cytosolic region a immunoreceptor tyrosine-based inhibitory motif (ITIM) supposed to be involved in the down-regulation of responses of phagocytic and antigen-presenting cells [22, 23]. The murine Siglec-E, an ortholog of human Siglec-9, binds to α-2,3- and α-2,6-linked sialic acids. In a non-activated state, Siglecs bind their ligands via cis-interactions on the same cell surface, because Siglecs are themselves sialylated lectins. If there is a ligand with a higher affinity, this binding is replaced by a trans-interaction [24, 25].
Another major virulence factor described in T. cruzi infection is the trans-sialidase (TS). It is known to be involved in infectivity and pathogenicity. This enzyme can transfer sialic acids from host cell glycoconjugates to mucin-like structures on the parasite surface. During infection, an immunodominant CD8+ T cell response against TS is generated. Previous results have shown that the trans-sialylation capacity is involved in the inhibition of the activation of antigen-presenting cells via Siglec-E interaction. This leads to a down-regulation of the Th1 response against the infection, favoring persistence of the parasite [26]. Interestingly, Jacobs and coworkers have demonstrated that sialylated structures from T. cruzi pathogenic Tulahuen (Tul) and to a less extent the less pathogenic Tehuantepec (Teh) strain interact with mouse Siglec-E [23]. The interaction between T. cruzi and Siglec-E resulted in a reduction of cytokine production as well as an unpaired T cell activation [23]. Therefore, taking into account that we have previously demonstrated the presence of sialic acid [19] and sulfated oligosaccharides [11] in Cz, that these sulfated structures play an important role in the immune response of the host [20], and that human Siglec-8 and Siglec-9 showed significant interactions with several sulfated sugars [27], and we evaluated the interaction between this sulfate-containing sialylated parasite molecule and mouse Siglec-E–Fc fusion molecules with the aim to investigate the involvement of sulfate moieties of Cz on the immunomodulatory effects of T. cruzi. The studies performed reflected the participation of the sulfated moieties of Cz in the Siglec-E–Fc interaction. In addition, a significant decrease in the binding of Siglec-E–Fc to parasites treated with increasing amounts of chlorate, a known inhibitor of the sulfation mechanism, was shown. The data presented in this work set the first report of sulfates as parasite ligands enhancing Siglec-E recognition. Herein, we also present data of Siglec-E binding to different virulent strains of the parasite and isolates from patients.
Methods
Parasites and strains
Epimastigotes were grown in axenic medium and harvested as described by Cazzulo et al. [28]. Hg39 cell monolayers grown in DMEM medium containing 10 % FCS were infected with trypomastigotes of T. cruzi strains Teh, Tul 2, Brazil and Y. Trypomastigotes were obtained free of cellular debris by leaving them to swim off the centrifuged pellet for 1 h at 37 °C [29]. Epimastigote lysates of the different T. cruzi strains (Tul 2, RA, Sylvio X10/7, DM28c and CA1-72) and parasite isolates [Fatala Chaben, Vazquez (San Luis), Motter (Formosa) and Venezuela], obtained in the facilities of INP, Dr M Fatala Chaben, ANLIS-Malbrán, Ministerio de Salud, Argentina, were tested for Siglec-E binding via ELISA.
Purification of IgGs
Precipitation of antibodies from rabbit sera was done with ammonium sulfate, and IgG was purified by passing over a Protein A–Sepharose column and eluting the adsorbed IgG with 0.1 M citrate buffer at pH 3.5 according to Ey et al. [30].
Purification of Cz and C-T domain
Cz was purified to homogeneity from epimastigotes of Tul 2 strain by affinity chromatography on concanavalin A–Sepharose followed by Mono Q chromatography step [20]. The C-T domain was obtained by self-proteolysis of highly purified Cz active preparations and purified by gel filtration in a Biogel P-30 column as previously described [19].
Production of rabbit sera specific for Cz and C-T and its desulfated counterparts (dCz and dC-T)
Rabbits (New Zealand white lineage) were immunized with Cz and C-T prior to and after desulfation, extracted from polyacrylamide gels (three doses of 50 µg protein intradermal via, in each case), as described [31]. Experimental procedures were conducted in the INP, “Dr Mario Fatala Chaben,” Ministerio de Salud, Argentina, in accordance with the ethical legislations and regulatory entities, established in Argentina and International Guides for care and use of laboratory animals.
Desulfation treatment
Samples were chemically desulfated as previously described [32].
Membrane extraction
Trypomastigotes (108–1010) were suspended in 10 mM Tris and 2 mM EDTA (TE) and washed twice in TE (spin at 40,000 × g for 8 min each time). After the addition of cells dropwise in 1 ml TE to 2 % Triton X-100 in PBS while stirring (final concentration 1 % Triton X-100), solubilization was performed during 30 min at 4 °C followed by an overnight incubation at the same temperature. After centrifugation at 100,000×g for 10 min, solubilized membrane proteins were recovered in the supernatants.
Subcellular fractionation
Epimastigotes of the Tul 2 stock were ground in a mortar with silicon carbide, and the nuclear (N), large granule (LG), small granule (SG), microsomal (M) and soluble (S) fractions were obtained by differential centrifugation as previously described [33].
Construction and preparation of Siglec-E–Fc chimera
Siglec-E–Fc chimera was obtained according to Erdman et al. [23]. A subclone stably expressing Siglec-E–Fc was routinely maintained in CEL Line bioreactors (IntegraBiosciences) with RPMI l-glutamine. Fusion containing 10 % IgG-depleted FCS and 2 mM protein secreted into medium was purified by HiTrap protein G columns (GE Healthcare) using standard protocols. Generation of anti-Siglec-E IgG was performed in accordance with previous study described by Erdmann et al. [23].
Expression of full-length Siglec-E in CHO cells
A full-length DNA fragment of Siglec-E was amplified from cDNA using specific primers. The fragment was cloned into the expression vector pcDNA3.1(+) (Invitrogen) and transfected into CHO cells by electroporation. Cells were subcloned and tested by flow cytometry for Siglec-E expression as previously described [23].
Purification of Cz by affinity to Siglec-E–Fc
Coupling of Siglec-E–Fc (100–200 µg) to activated BrCN Sepharose-4B (300 mg/ml) was performed using standard protocols for preparation of affinity columns. Supernatants from spontaneously generated metacyclic trypomastigotes [34] were dialyzed against 50 mM Tris–ClH pH 7.6 and applied onto the Siglec-E–Sepharose column (1 ml) three times, washed and consecutively eluted with 0.5 M and 3 M NaCl. Purification steps were tested by SDS-PAGE followed by silver staining and Western blot with rabbit polyclonal sera specific for Cz.
SDS-PAGE with or without gelatin
Purification was followed by 10 % SDS-PAGE using the discontinuous buffer system described by Laemmli [35], and minigels were stained with silver nitrate [36]. Detection of proteinase activity in lysates from T. cruzi epimastigotes was performed in 10 % resolving SDS-acrylamide gels containing 0.15 % copolymerized gelatin as previously described by Duschak et al. [37]. Protein content was measured by Lowry’s method [38]. Protein content was measured by Bradford’s method [39].
Western blotting
Samples submitted to SDS-PAGE were electrotransferred to nitrocellulose membranes for 2 h at 200 mA. After blotting, nitrocellulose sheets were coated with Tris-buffered saline solution containing 3 % nonfat power milk (TBS-M). The transferred proteins were incubated with polyclonal rabbit sera specific for Cz (1/1000 dilution in TBS-M), incubated with peroxidase mice anti-rabbit IgG (1/2000 in TBS-M) and developed with a 4-chloro-1-naphthol in methanol/PBS solution [40].
Flow cytometry staining
For interaction analysis between IgGs and T. cruzi strain Tul 2, rabbit IgGs specific for Cz, dCz, C-T and dC-T were purified and pre-immune rabbit IgGs were used as control. For staining protocol, the trypomastigotes were washed with 0.1 % FCS in PBS (FACS buffer) and incubated separately with 10 µg of each specific IgG for 60 min. After another washing step with FACS buffer, trypomastigotes were incubated with a FITC-labeled mouse anti-rabbit antibody (1/100) for 40 min. Then, the samples were washed twice with FACS buffer and fixed with 2 % PFA. Samples were loaded at C6 flow cytometer. Strains: Tul 2, Teh, Y and Brazil.
Flow cytometry staining for lectin interactions analysis included an initial pre-incubation step of each lectin with the corresponding secondary antibody. For pre-incubation, each sample contained 1 µg of biotinylated Mal II + streptavidin APC in PBS (1/40) and 2 µg of Siglec-E–Human Fc + donkey antihuman phycoerythrin in PBS (1/40). After pre-incubation for 30–60 min, 10 µl of the mentioned solution was added to each sample of cleaned trypomastigotes (in ~90 µl of FACS buffer) and kept for at least 1 h 30 min. After the incubation, the samples were washed twice with FACS buffer and fixed with 2 % PFA.
ELISA and ELISA competitive assays
ELISA was used to determine the binding capacity of either Siglec-E–Fc or Mal II to T. cruzi strains and to evaluate the ability of different T. cruzi biomolecules to interact with Siglec-E–Fc. The molecules were fixed to high-binding plates and then stained first with Siglec-E–Fc and second with a peroxidase conjugated antihuman Fc antibody. Biotinylated Mal II (Maackia amurensis lectin II, specific for α-2-3-linked sialic acid) was used as control to detect the presence of sialic acid in the diverse molecules. In all cases, bovine serum albumin (BSA) was used as negative control. Both Siglec-E–Fc and Mal II secondary antibodies were used as negative controls.
Competitive assays between Siglecs and Cz were performed using a constant amount of dCz (1 µg) as antigen and growing amounts of competitor Cz (0, 0.1, 0.5 and 1 µg). Cz activity was inhibited with E-64 100 µM for 30 min at 0 °C for these assays in reducing conditions (DTT 0.1 M). Another competitive assay was performed using 1 µg microsomal fraction as antigen and confronted with increasing amounts (0 y 1 and 2 µg) either Cz or desulfated Cz. In both cases, recognition by Siglec-E–Fc was performed as above described.
IFI
T. cruzi trypomastigote forms from Tul 2 strain were incubated with Siglec-E–Fc followed by incubation with a FITC-labeled antihuman IgG antibody. Slides were mounted in buffered glycerin and visualized by fluorescence microscopy with a magnification of 400×.
Chlorate treatment
Trypomastigotes from Brazil strain of T. cruzi were used to infect Hg39 cells (human glioblastoma cell line 39) at different concentrations of sodium chlorate (0, 10, 20 and 40 mM). The cell culture medium contains 75 % Dulbecco’s Modification of Eagle’s Medium (MP Biomedicals, LLC), 25 % of IMDM medium (Gibco) and 2.5 % FCS for the first 72 h. The last 24 (day four) hours when the resulting parasites were harvested the cell culture medium was 100 % Dulbecco’s Modification of Eagle’s Medium supplemented with 1 % FCS. Every 24 h the medium was renewed and fresh sodium chlorate was added [41]. The harvested parasites were stained with either Mal II or Siglec-E–Fc according to the procedures above described.
Statistical analysis
Results are presented as the mean standard deviation. Each experiment was performed independently three to four times. Statistical analysis was generally performed with the one-way or two-way ANOVA test using PRISM software (GraphPad Software, San Diego, CA, USA). For Mal II recognition ELISA, one-way ANOVA was used. For Siglec-E recognition, two-way ANOVA test was used. The level of significance was set at *P 0.05 and **P 0.01. ***P 0.0001.
Results
Cz gelatinolytic activity and differential binding of mouse Siglec-E lectin to epimastigotes from virulent Trypanosoma cruzi strains and to trypomastigotic Tulahuen 2
Epimastigote lysates of five virulent T. cruzi strains were tested for gelatinolytic activity in SDS-PAGE (Fig. 1A) and for Siglec-E–Fc-binding capacity by ELISA (Fig. 1B) showing differences. The gelatinolytic activity of Tul 2 and DM28c (Fig. 1A, a and b, respectively) is similar to and higher than that from CAI-72 (Fig. 1A, d), respectively. The lowest activity was observed in strains RA and Sylvio X10/7 (Fig. 1A, b and e, respectively). In addition, Sylvio X10/7 activity bands showed lower mobility and RA faster mobility than the other virulent strains tested (Fig. 1A, e vs b). Accordingly, binding affinity to Siglec-E–Fc of the parasite strains tested showed to be higher for Tul 2 and DM28c than for CAI-72, RA and Sylvio X10/7 strains. Parasite strains showed a different type of affinity to Siglec-E–Fc, which could be divided into high to intermediate binders (DM28c, Tul 2, CAI/72) and low binders (RA and Sylvio) among the strains tested. In addition, binding affinity to Siglec-E–Fc was tested on patient parasite isolates, showing differential binding too (Fig. 1C). On the other hand, Siglec-E–Fc binding to trypomastigotic Tulahuen 2 strain was visualized by immunofluorescence imaging with a FITC-labeled antihuman IgG antibody. IFI assays confirmed the binding of Siglec-E–Fc to trypomastigote surface proteins (Fig. 1D).
Differences in Cz activity and binding of mouse Siglec-E–Fc lectin to epimastigotes of different T. cruzi strains, to patient isolates and to trypomastigotes from Tulahuen 2 strain. A Cz activity in 10 % SDS-PAGE using 0.15 % gelatin as substrate for lysates (1 × 106 epimastigotes) from a Tulahuen II, b RA, c DM28c, d CAI/72 and e Sylvio X10/7. Gels were incubated for 2 h at 37 °C in buffer MES 0.1 M pH 6 in the presence of DTT 0.5 mM followed by staining with Coomassie Brilliant Blue 0.2 %. B ELISA results showing differential Siglec-E–Fc binding to five T. cruzi strain lysates (Tulahuen II, RA, DM28c, CAI/72 and Sylvio X10/7). The background represents the Siglec binding to the blocking buffer. Each bar shows mean ± SD. C ELISA results showing differential Siglec-E–Fc binding to lysates of four patient isolates obtained and named in the facilities of INP, Dr M Fatala Chabén, ANLIS-Malbrán, Ministerio de Salud, Argentina as: I—Fatala Chaben, II—Vazquez (San luis), III—Motter (Formosa) and IV—Venezuela. D Siglec-E–Fc binding to trypomastigotes of T. cruzi Tul 2 strain was visualized by immunofluorescence imaging with an FITC-labeled antihuman IgG antibody (b) in comparison with the negative control (a)
Microsomal fraction contains Siglec-E-binding proteins
In order to get an insight into the localization of the proteins that bind to Siglec-E, we performed subcellular fractionation experiments by differential centrifugation of epimastigote homogenates obtained after disruption by grinding with silicon carbide. The marker enzyme patterns were as previously described [42], indicating that the large granule (LG) and small granule (SG) fractions consisted of mitochondrial vesicles, lysosomes and glycosomes to a different extent, and the microsomal (M) fraction was essentially made up of endoplasmic reticulum and plasma membrane fragments. Also, nuclear (N) and soluble (S) fractions were tested. Interestingly, the enriched membrane-containing microsomal fraction clearly showed the highest amount of sialic acid (Fig. 2A). As expected, microsomal fraction showed the highest Siglec-E–Fc binding among the fractions tested (Fig. 2B). For the other fractions, the binding of both lectins was barely detectable.
Increased binding of Siglec-E to proteins of the microsomal fraction. A Siglec-E–Fc binding to growing amounts (0.5, 1 and 2 µg of total protein) of the different subcellular fractions of T. cruzi epimastigote homogenates was tested. Fractions were obtained after parasite rupture with silicon carbide followed by differential centrifugation. The value of the BSA (negative control) was properly subtracted from the sample. B Equal amounts (1 μg) of the different subcellular fractions were tested via ELISA for sialic acid content with α-2,3-specific lectin, Mal II (Maackia amurensis lectin II). The proteins of the microsomal fraction of parasite lysates show a high binding to Siglec-E. This correlates with the Mal II recognition and the sialic acid amount. Fractions: nuclear (N), microsomal (M), small granules (SG), large granules (LG) and soluble (S). Statistical analysis was performed by using one- and two-way ANOVA test in A and B, respectively, ***p < 00001. C Membrane proteins were extracted from the strains Tul and Teh with Triton X-100 from 1 × 106 parasites, and comparable amounts were confronted with Siglec-E and Mal II lectin for sulfates and for sialic acid recognition, respectively, by ELISA. In all cases, ELISA was performed using sample duplicates
Different binding capacity of Siglec-E to membrane-bound proteins obtained from trypomastigotes of two strains with different degree of virulence
Binding of membrane proteins from trypomastigotes obtained by extraction with Triton X-100 1 % from the pathogenic strain Tul 2 to Mal II and Siglec-E–Fc was compared with the less virulent strain Teh (Fig. 2C). These results are in accordance with previous data obtained by flow cytometry using the same parasite strains [23].
Presence of sulfates of Cz/C-T indicated by flow cytometry on the surface of trypomastigotes from different T. cruzi strains
In order to further elucidate the role of sulfated epitopes in the interaction between membrane-bound Cz from trypomastigotes of T. cruzi and Siglec-E, fluorescence-activated cell sorting (FACS) was used to determine the binding capacity of anti-Cz IgGs purified from polyclonal rabbit sera to T. cruzi trypomastigotes in comparison with those purified from polyclonal sera specific for the desulfated forms of Cz (Fig. 3). In order to verify whether these results are common to different strains, flow cytometry analysis was performed with trypomastigotes from T. cruzi strains Tul 2, Brazil, Y and Teh. IgG antibodies specific for Cz prior to (3I A, C, E and G) and after (3I B, D, F, and H) desulfation treatment showed that in all cases, only a part of the parasites population expresses Cz and that a higher population of parasites was recognized by antibodies specific for Cz as compared to the recognition observed by antibodies specific for the desulfated protein. This result demonstrates that sulfated moieties of Cz are exposed as epitopes over the parasite surface (Fig. 3II). Similar results were obtained with the T. cruzi strains Teh and Y with IgGs specific for C-T prior to (Fig. 4I, A, C; II) and after (Fig. 4I, B, D, II) desulfation treatment, in line with the presence of sulfates in the C-T domain.
Recognition of sulfated antigens confronting Trypanosoma cruzi trypomastigotes from different strains. I Tul 2, Brazil, Y and Teh, and strains tested with IgGs specific for Cz (A, C, E, G) and dCz (B, D, F, H), respectively, by fluorescence-activated cell sorting (FACS). II Graph bar indicating recognition (%) of trypomastigotes from Tul 2, Brazil, Y and Teh strains
Recognition of sulfated antigens confronting Trypanosoma cruzi trypomastigotes from different strains: I Y and Teh strains with IgGs specific for C-T (A, C) and desulfated C-T (B, D), respectively, by fluorescence-activated cell sorting (FACS). II Graph bar indicating recognition (%) of trypomastigotes from Y and Teh strains
Siglec-E and Mal II competition by the same ligand on Trypanosoma cruzi
It is noteworthy that we do not suggest that sulfates are primary receptors for Siglec-E. Knowing that similar to Siglec-E, Mal II also binds α-2,3-linked sialic acid, we used flow cytometry to perform competition assays to test whether the sialic acid-binding lectin Mal II and Siglec-E–Fc compete for similar binding sites. For that purpose, trypomastigotes were fixed and stained with either Siglec-E–Fc alone, Mal II alone, or Siglec-E–Fc followed by Mal II. In Fig. 5A, the staining intensity of parasites with Siglec-E–Fc is depicted, showing that staining intensity of Siglec-E–Fc decreased upon addition of Mal II in comparison with the intensity obtained when the staining was performed with Siglec-E alone. Figure 5B shows that Mal II binding is not prevented by pre-incubation with Siglec-E–Fc because the same Mal II-binding intensity is obtained in the absence of competition. These experiments indicate that Mal II and Siglec-E–Fc compete for the same ligands on the parasite, although Mal II exhibited a higher affinity than Siglec-E–Fc. The residual Siglec-E–Fc binding in Fig. 5A after competition may be attributed to the fact that Siglec-E also binds α-2,6-linked sialic acids while Mal II does not.
Siglec-E–Fc and Mal II competition assay to evidence different binding affinities to shared parasite ligands on Trypanosoma cruzi. Fixed parasites were stained either with Siglec-E–Fc, or with Mal II, or with Siglec-E–Fc followed by Mal II. Staining of these sialic acid-binding reagents was detected using the appropriate detection reagents for bound Siglec-E–Fc (A) or bound Mal II (B) and was analyzed by flow cytometry. Similar results were obtained in two independent experiments
Interaction between membrane-bound isoforms of Cz and Siglec-E–Fc
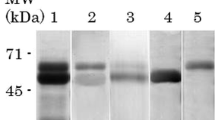
In order to confirm whether secreted Cz from T. cruzi trypomastigotes is capable to bind to Siglec-E, supernatants from CL metacyclic trypomastigotes, containing secreted Cz isoforms [33], were subjected to Siglec-E–Fc affinity chromatography. Different isoforms of Cz bind specifically to the column as verified by SDS-PAGE followed by silver staining and immunoblotting with rabbit polyclonal sera specific for Cz of eluted material (Fig. 6A, B). This confirms that membrane-bound isoforms of Cz are capable to bind to Siglec-E–Fc.
Purification of membrane-bound isoforms of Cz by affinity chromatography using Siglec-E–Fc. A SDS-PAGE followed by silver staining. Supernatant obtained from spontaneous differentiation CL Brener metacyclic trypomastigotes (1), Siglec-E–Fc column flow through (2), Siglec-E–Sepharose column eluate (3), Cz (4), 5 (BSA). B Western blot revealed with rabbit anti-Cz serum. Supernatant obtained from spontaneous differentiation CL Brener metacyclic trypomastigotes (1), Siglec-E-column flow through (2), Siglec-E–Sepharose column eluate (3), Cz (4)
Involvement of sulfate groups from N-glycans present in the C-T domain of Cz in Siglec-E binding
In order to investigate whether sulfated moieties of T. cruzi glycoproteins participate in Siglec-E binding, Cz and C-T prior to and after desulfation treatment were subjected to binding ELISA assays using mouse Siglec-E–Fc fusion molecules. Sialic acid recognition was tested using the Mal II lectin as a control. Mal II recognition of dCz was significantly higher compared to non-treated Cz. Taking into account that desulfation of Cz could not increase the sialic acid content, we suggest that the desulfation process might produce protein denaturalization with the consequent of a higher exposure of sialic acid-containing oligosaccharide chains. To test this possibility, we performed a denaturing treatment of Cz, demonstrating that interaction with Mal II is increased after the partial denaturation of the molecule (Fig. 7A). However, taking into account that dCz and dC-T showed a higher binding to Mal II than the sulfated forms despite the treatment (Fig. 7B), dCz and dC-T were considered as 100 % of sialic acid exposition and used to calculate the relative binding from Siglec-E–Fc to Cz prior to and after desulfation treatment (Fig. 7C). While a significant difference in Siglec-E–Fc binding between Cz and dCz was observed at all the protein concentrations tested, the significant decrease in recognition of Siglec-E–Fc for C-T and dC-T was observed when the ELISA was performed using 1 µg of each protein. These results indicate a significant contribution of the sulfated moieties to the binding between Siglec-E–Fc and this glycoprotein (Fig. 7C).
Involvement of sulfate groups from N-glycans present in the C-T domain of Cz in Siglec-E binding. Recognition of Mal II lectin of A native Cz, denatured Cz and desulfated Cz (dCz); B Cz and C-T domain with (dCz, dC-T) and without (Cz, C-T) desulfation treatment. The results are the average of three ELISAs by triplicates. The values obtained were used to establish the relation between Mal II recognition (exposed sialic acid) and Siglec-E binding. Denaturizing protocol: 100 µl of Cz (0.1 μg/µl) + 2 μl of 2-mercaptoethanol during 100 °C for 5 min. Statistical analysis was performed by using one-way ANOVA test *p < 0.05; **p < 0.01; ***p < 0.000; C ratio between Mal II recognition and Siglec-E–Fc-binding values of different amounts (0.1, 0.5 and 1 µg) of Cz and C-T with (dCz, dC-T) and without (Cz, C-T) desulfation treatment. Mal II-binding values from dCz in B were considered as 100 % of sialic acid exposition and used to obtain the relative value of Siglec-E binding for sulfated and desulfated molecules in accordance with their sialic acid exposure in B
Involvement of sulfates on host–parasite relationship by the interaction of Cz and Siglec-E–Fc
In order to confirm the involvement of sulfate groups in the interaction of Cz and Siglec-E–Fc, a competitive binding assay between Cz prior to and after desulfation treatment and Siglec-E–Fc was performed using dCz as antigen and increasing amounts of Cz in conjunction with a constant amount of Siglec-E–Fc. A complete inhibition of the interaction dCz–Siglec-E confirmed the involvement of sulfated epitopes in the interaction between this sulfated glycoprotein and the mentioned IgG like-lectin (Fig. 8A).
Involvement of sulfates in the interaction between membrane-bound isoforms of Cz and Siglec-E–Fc. A Competitive inhibition of ELISA assay between Cz and dCz for binding to Siglec-E using 1 µg dCz as antigen bound on the plate. Incubation with Siglec-E–Fc in conjunction with increasing amounts of Cz (0, 0.1, 0.5, and 1 µg) was performed. The plate was developed with an antihuman Fc HRP antibody. B Competitive ELISA assay was performed using 1 µg microsomal fraction as antigen and confronted with increasing amounts (0 y 1 and 2 µg) of either Cz or desulfated Cz. In both cases, recognition by Siglec-E–Fc was performed as described in materials and methods
In addition, it was tested whether the protein environment contributes to the Siglec-E binding to the sulfated glycoprotein. A competitive assay was performed by fixing proteins from the Cz-containing parasite microsomal fraction to the plate, while purified Cz and dCz were used as competitors. The addition of Cz to the plate containing proteins from the microsomal fraction did not alter the absorbance values obtained. However, binding to Siglec-E–Fc increased significantly when dCz was used as ligand, suggesting that Siglec-E binding to sialic acid-containing proteins from microsomal fraction was favored when dCz was used as competitor, indicating that the protein environment might modify Siglec-E affinity to natural ligands (Fig. 8B).
Binding of Mal II and Siglec-E to trypomastigotes treated with chlorate
Alternatively, in order to confirm whether the parasite sulfation rate can affect the Siglec-E–Fc recognition of the parasite surface, we used sodium chlorate, which is known to inhibit sulfation process. Chlorate specifically inhibits ATP sulfurylase, a key enzyme in the PAPS (3′-phosphoadenosine 5′-phosphosulfate) sulfation pathway [43, 44]. We have previously shown that the PAPS sulfation pathway is present in T. cruzi using sodium chlorate as a sulfation inhibitor [41]. Trypomastigotes from Brazil strain of T. cruzi were grown at increasing concentrations of sodium chlorate (0, 10, 20 and 40 mM) in Hg39 cells. After the treatment, the binding of Siglec-E–Fc was measured in order to evaluate the participation of the sulfates from the parasite surface ligands in this interaction. It is worth noting that not only Cz but also sulfatides or any sulfated molecule is able to be affected by chlorate treatment. However, purified sulfatides do not bind to Siglec-E (data not shown), but a possible binding to another unknown sulfated molecule that might cause an alteration in the Siglec-E recognition cannot be discarded. Mal II staining of these treated parasites was used as a sialylation rate control. While the recognition of sialic acid, main ligand of Siglecs over the parasites surface remains constant during the treatment (Fig. 9E–H, II), and the interaction of Siglec-E with the parasite decreases as the chlorate concentration increases (Fig. 9A–D, I). Thus, chlorate treatment results confirm the participation of sulfates in the Siglec-E recognition of its cognate ligands. Same results were obtained with Tulahuen strain of T. cruzi (data not shown).
Decreased binding from Siglec-E to parasites treated with growing amounts of chlorate confirms the participation of sulfates in this interaction. Dot plots from the flow cytometry binding analysis of chlorate-treated Brazil strains parasites, from T. cruzi, to Siglec-E and Mal II lectins. Binding of untreated control parasites (without sodium chlorate) (A, E), parasites treated with 10 mM (B, F), 20 mM (C, G) and 40 mM (D, H) of sodium chlorate Siglec-E–Fc and Mal II, respectively. The positive events of the mentioned dot plots were represented in a bar graph (I, II), respectively. Statistical analysis was performed by using one-way ANOVA test followed by Dunnett post test *p < 0.05 in Graphpad Prism
Discussion
Sulfation is a critical modification in biological recognition. Sulfated oligosaccharides play diverse relevant roles in binding to receptors and mediating adhesion to the cell surface [45–47]. In T. cruzi, sulfated structures have been described as part of glycolipids representing common antigens on the surface of the parasite and mammalian cells [48–50]. The presence of sulfate groups in N-linked oligosaccharides has been reported in virus [51] and in mammalian cells [52], mainly implicated in specific molecular recognition processes [53, 54]. In T. cruzi, first evidences pointed out to the presence of an acidic component as part of Cz: The presence of up to 12 bands with pI values ranging from 3.7 to 5.1 was detected by isoelectrofocusing [55]. The pI values determined for purified C-T ranged from 4.5 to 5.0 [8], in contrast to the pI values calculated from the amino acid sequences of the C-T in different Cz isoforms ranging from 7.05 to 8.12 [56]. Phosphate in the mature enzyme, which could account for this discrepancy, had been discarded by in vivo labeling of the parasites with 32P [57]. Noticeable, we have shown for the first time the presence and the antigenic properties of sulfated high-mannose-type oligosaccharides present in the C-T domain of Cz [11, 20]. Our results in conjunction with those reported in Dyctiostelium discoydeum are, as far as we know, the only ones describing the antigenic properties of this type of structures [20, 58]. On the other hand, sera from chronically T. cruzi-infected subjects with mild disease displayed higher levels of IgG2 antibodies specific for sulfated epitopes compared with those in more severe forms of the disease, suggesting a potential role for sulfate moieties in the control of chronic T. cruzi infection [20, 31]. Recently, we found that synthetic anionic sugar conjugates containing GlcNAc6S competitively inhibit the binding of affinity-purified rabbit anti-C-T IgG to the C-terminal extension of Cz. Interestingly, extending these findings to the context of natural infection, immune assays performed with Chagas disease serum confirmed that the structure of synthetic GlcNAc6S mimics the N-glycan-linked sulfated epitope displayed in the C-T domain of Cz [59]. However, no studies about the possible participation of these groups enhancing host–parasite interaction via Siglec-E binding have been studied so far.
Although binding of Siglecs critically depends on sialic acid, strong differences in binding to various sialylated ligands were found using glycoarrays. Therefore, in order to investigate whether sulfated moieties might be involved in the immunomodulatory effects of T. cruzi, several techniques using mouse Siglec-E–Fc fusion molecules and parasite Cz and C-T prior and after desulfation treatment were performed, showing a significant binding decrease after desulfation treatment of these molecules. Our findings, in accordance with glycoarray data from the glycoconsortium (http://www.functionalglycomics.org/glycomics/publicdata/selectedScreens.jsp), indicate that the presence of sulfates in sialylated glycoproteins might enhance the interaction with Siglec-E, an ortholog of human Siglec-9, and thereby modulate the immune response of the host, favoring the persistence of the parasite in the organism.
Taking into account that epimastigotes and trypomastigotes of three attenuated T. cruzi sublines displayed a weaker gelatinolytic activity of Cz than their virulent ancestor strains [37], Cz activity might be considered as a possible pathogenicity marker. Herein, we analyzed Cz activity of five virulent strains. This activity showed to be higher for Tul 2 and DM28c than for CAI-72, which had a intermediate activity, while RA and Sylvio X10/7 strains showed the lowest activity among them. These differences can account for the expression of different number of genes among the virulent strains tested under the knowledge that the major protease of the parasite is encoded by multiple polymorphic tandemly organized genes [60]. Surprisingly, the same binding order, from high to low binding, to Siglec-E–Fc was obtained by these same strains, suggesting a possible association between virulence degree and Siglec-E binding [23]. IFI assays confirmed the binding of Siglec-E to trypomastigote surface proteins.
Proteins of the microsomal fraction of T. cruzi lysates showed the highest binding to Siglec-E in comparison with nuclear, large granules, small granules and soluble parasite fractions. The membrane-bound proteins are clearly the most sialylated ones in the parasite. This is thought to be a strategy for host immune response evasion, and our results agree with it. We assume that the signal obtained within the soluble fraction may be due to some contamination with another fraction, because there was no positive signal when this enriched, but not highly purified subcellular fraction was tested with Mal II lectin. The recovery of Cz isoforms from supernatants of metacyclic forms with a Siglec-E–Sepharose affinity chromatography, in line with the results of the ELISA competitive assays, confirms that Cz interacts with Siglec-E–Fc.
The results obtained by flow cytometry with Tul 2 trypomastigotes stained with Mal II lectin and Siglec-E–Fc simultaneously corroborated the finding that both Siglec-E–Fc and Mal II lectin bind to α-2,3-linked sialic acids, indicating that Mal II and Siglec-E–Fc compete for the same ligands on the parasite. However, Mal II reduces the binding of Siglec-E–Fc, exhibiting a higher affinity than Siglec-E–Fc for its ligands.
Sulfated antigens were differentially recognized with purified IgGs from polyclonal rabbit sera specific for Cz/dCz and C-T/dC-T. It can be suggested that the differences between Cz recognition in the different strains may be due to differences between Cz isoforms among different strains, taking into account (1) that IgGs were obtained from sera of rabbits immunized with Cz purified from epimastigotes of Tul 2 strain, (2) that the antibodies are directed toward the lysosomal form, (3) that the different strains contain different number of Cz genes and (4) that these isoforms can also contain differences in sulfated epitopes. The significant decrease in binding assays of dCz and dC-T to Siglec-E showed that sulfates play an important role in Siglec-E interaction with its ligand, suggesting that sulfates enhance this interaction. The percentage of recognition of Cz and dCz with specific antibodies showed to be similar between Tul 2 and Teh, indicating a similar contribution of sulfates in the binding of Cz to Siglec-E in both strains. This result suggests that sulfate ligands should not be involved in the difference of virulence between both strains. Thus, this difference must be due to sialic acid [23]. While the Y strain showed a lower percentage of recognition of sulfates than Tul 2 and Teh, Brazil strain presented the lowest recognition values, half of which correspond to sulfates. Herein, flow cytometry analysis revealed a specific and significant immune recognition against sulfated moieties as antigens on the trypomastigote surface.
On the other hand, inhibition of sulfation mechanism using chlorate strongly supports that sulfates on Siglec-E ligands like Cz enhance the interaction. Taking into account that the level of interaction determines the effect of Siglec binding and Siglecs are known to participate in the modulation of the immune response, we can infer that sulfates might indirectly have a role as immunomodulators over T. cruzi trypomastigotes through Siglec signaling. Further studies must be performed to elucidate whether the differential expression of sulfated glycoproteins in the different developmental stages might be a requirement for parasite immunomodulation along the life cycle of the parasite. The promissory results obtained based on Siglec-E–Cz binding, as well as the involvement of sulfated structures in this host–parasite interaction will allow to study the immunomodulatory effect of T. cruzi on phagocytic and antigen-presenting cells. The role of these epitopes in the innate immune response and in the dissemination of the parasite may help to better understand the biology of T. cruzi and the mechanism of parasite infection.
Conclusions
Siglec-E and its ortholog human protein, Siglec-9, are well known as immune system modulators. Mainly, in innate immune cells, the trans-interaction of these lectins with their ligands down-regulates cell activation and/or proliferation [22, 23, 61]. Some evidences support that sulfates enhance the binding of Siglec-9 probably promoting the trans-interaction and signaling activation [27, 62]. It is also described that Siglec-E also binds to sulfated saccharides [22, 26] and that sulfation might be responsible for the interaction of innate immune cells which express Siglec-E, with specific T cells upon their activation, leading to the activation suppression of the first ones [63]. We have previously reported the presence of sulfated oligosaccharides on the unique N-glycosylation site of the C-T domain of Cz, the major cysteine proteinase of T. cruzi [11], and showed that these sulfate moieties are targets for specific immune responses [20]. Interestingly, we also found that subjects chronically infected with T. cruzi mount specific IgG2 humoral immune responses to this sulfated glycoprotein which inversely correlate with human severe chronic Chagas disease [20, 21].
In this work, we have demonstrated that Cz binds to Siglec-E and this interaction is boosted with the sulfation of the protein. We also show that the sulfated epitopes of Cz, probably among others, are exposed over the parasite surface and that sulfation inhibition with sodium chlorate negatively affects Siglec-E recognition of trypomastigote surface. Besides, we have also shown that Siglec-E interacts with T. cruzi trypomastigotes and this interaction might correlate with the virulence as previously described [23]. Altogether, our findings support not only the notion that Siglec-E ligand recognition is enhanced by the presence of sulfates, but also that T. cruzi sulfation has a determinant role in immunomodulation of the host response upon the infection. The present discovery along with the ones previously described by our research groups denotes the huge importance of Cz sulfation in the evolution of Chagas disease.
References
WHO (2010) Available at: http://www.who.int/mediacentre/factsheets/fs340/en/index.html
Gascon J, Bern C, Pinazo MJ (2010) Chagas disease in Spain, the United States and other non-endemic countries. ActaTrop 115:22–27 Review
Pérez-Molina JA, Norman F, López-Vélez R (2012) Chagas disease in non-endemic countries: epi-demiology, clinical presentation and treatment. Curr Infect Dis Rep 14:263–274
Scharfstein J, Rodriguez MM, Alves CA, de Souza W, Previato JO, Mendonca-Previato L (1983) Trypanosoma cruzi: description of a highly purified surface antigen defined by human antibodies. J Immunol 131(2):972–976
Scharfstein J, Luquetti A, Murta AC, Senna M, Rezende JM, Rassi A, Mendonça-Previato L (1985) Chagas’ disease: serodiagnosis with purified Gp25 antigen. Am J Trop Med Hyg 34(6):1153–1160
Martinez J, Campetella O, Frasch ACC, Cazzulo JJ (1991) The major cysteine proteinase (cruzipain) from is antigenic in human infections. Infect Immun 59(11):4275–4277
Martinez J, Campetella O, Frasch ACC, Cazzulo JJ (1993) The reactivity of sera from chagasic patients against different fragments of cruzipain, the major cysteine proteinase from Trypanosoma cruzi, suggests the presence of defined antigenic and catalytic domains. Immunol Lett 35:191–196
Cazzulo JJ, Labriola C, Parussini F, Duschak VG, Martinez J, Franke de Cazzulo BM (1995) Cysteine proteinases in Trypanosoma cruzi and other Trypanosomatid parasites. Acta Chim Slov 42:409–418
Cazzulo JJ, Hellman U, Couso R, Parodi AJA (1990) Amino acid and carbohydrate composition of a lysosomal cysteine proteinase from Trypanosoma cruzi. Absence of phosphorylated mannose residues. Mol Biochem Parasitol 38:41–48
Parodi AJA, Labriola C, Cazzulo JJ (1995) The presence of complex-type oligosaccharides at the C-terminal domain glycosylation site of some molecules of cruzipain. Mol Biochem Parasitol 69:247–255
Barboza M, Duschak VG, Fukuyama Y, Nonami H, Erra-Balsells R, Cazzulo JJ, Couto AS (2005) Structural analysis of the N-glycans of the major cysteine proteinase of Trypanosoma cruzi. Identification of sulfated high-mannose type oligosaccharides. FEBS J 272:3803–3815
Scharfstein J, Schmitz V, Morandi V, Capella MM, Lima AP, Morrot A, Juliano L, Müller-Esterl W (2000) Host cell invasion by Trypanosoma cruzi is potentiated by activation of bradykinin B(2) receptors. J Exp Med 6(192):1289–1300
Benítez-Hernández I, Méndez-Enríquez E, Ostoa P, Fortoul T, Ramírez JA, Stempin C, Cerbán F, Soldevila G, García-Zepeda EA (2010) Proteolytic cleavage of chemokines by Trypanosoma cruzi’s cruzipain inhibits chemokine functions by promoting the generation of antagonists. Immunobiology 215:413–426
Doyle PS, Zhou YM, Hsieh I, Greenbaum DC, McKerrow JH, Engel JC (2011) The Trypanosoma cruzi protease cruzain mediates immune evasion. PLoS Pathog 7(9):1–11
Duschak VG, Couto AS (2009) Cruzipain, the major cysteine protease of Trypanosoma cruzi: a sulfated glycoprotein antigen as relevant candidate for vaccine development and drug target. Curr Med Chem 16:3174–3202 Review
Cazorla SI, Frank FM, Malchiodi EL (2009) Vaccination approaches against Trypanosoma cruzi infection. Expert Rev Vaccines 8:921–935 Review
Cazzulo JJ, Stoka V, Turk V (2001) Cruzipain, the major cysteine proteinase from the protozoan parasite Trypanosoma cruzi. Curr Pharm Des 7:1143–1156
Duschak VG (2011) A decade of targets and patented drugs for chemotherapy of chagas disease. Recent Pat Antiinfect Drug Discov 6:216–259
Barboza M, Duschak VG, Cazzulo JJ, Lederkremer RM, Couto AS (2003) Presence of sialic acid in N-linked oligosaccharide chains and O-linked N-acetylglucosamine in cruzipain, the major cysteine proteinase of Trypanosoma cruzi. Mol Biochem Parasitol 126:293–296
Acosta DM, Arnaiz MR, Esteva MI, Barboza M, Stivale D, Orlando UD, Torres S, Laucella SA, Couto AS, Duschak VG (2008) Sulfates are main targets of immune responses to cruzipain and are involved in heart damage in BALB/c immunized mice. Int Immunol 20:461–470
Acosta DM, Soprano LL, Ferrero MR, Esteva MI, Riarte AR, Couto AS, Duschak VG (2012) Structural and immunological characterization of sulfatides: relevance of sulfate moieties in Trypanosoma cruzi glycoconjugates. Parasite Immunol 34:499–510
Crocker PR, Paulson JC, Varki A (2007) Siglecs and their roles in the immune system. Nat Rev Immunol 7:255–266 Review
Erdmann H, Steeg C, Koch-Nolte F, Fleischer B, Jacobs T (2009) Sialylated ligands on pathogenic Trypanosoma cruzi interact with Siglec-E (sialic acid-binding Ig-like lectin-E). Cell Microbiol 11:1600–1611
Crocker PR (2005) Siglec in innate immunity. Curr Opin Pharmacol 5:431–437 Review
Varki A, Angata T (2006) Siglecs, the major subfamily of type-1 lectins. Glycobiology 16(1):1–27 Review
Jacobs T, Erdmann H, Fleischer B (2010) Molecular interaction of Siglecs (sialic acid-binding Ig-like lectins) with sialylated ligands on Trypanosoma cruzi. Eur J Cell Biol 89:113–116
Rapoport EM, Pazynina GV, Sablina MA, Crocker PR, Bovin NV (2006) Probing sialic acid binding Ig-like lectins (siglecs) with sulfated oligosaccharides. Biochemistry (Mosc) 71:496–504
Cazzulo JJ, Franke de Cazzulo BM, Engel JC, Cannata JJ (1985) End products and enzymes levels of aerobic glucose fermentation in Trypanosomatids. Mol Biochem Parasitol 16:329–343
Andrews NW, Colli W (1982) Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. J Protozool 29:264–269
Ey PL, Prowse SJ, Jenkin CR (1978) Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Immunochemistry 15:429–436
Acosta DM, Soprano LL, Ferrero M, Landoni M, Esteva MI, Couto AS, Duschak VG (2011) A striking common O-linked N-acetylglucosaminyl moiety between cruzipain and myosin. Parasite Immunol 33:363–370
Freeze HH, Yeh R, Miller AL, Kornfeld S (1983) Structural analysis of the asparagine-linked oligosaccharides from three lysosomal enzymes of Dictyostelium discoideum. Evidence for an unusual acid-stable phosphodiester. J Biol Chem 258(24):14874–14879
Parussini F, Duschak VG, Cazzulo JJ (1998) Membrane-bound cysteine proteinase isoforms in different developmental stages of Trypanosoma cruzi. Cell Mol Biol 44:513–519
Duschak VG, Barboza M, Garcia GA, Lammel EM, Couto AS, Isola EL (2006) Novel cysteine proteinase in Trypanosoma cruzi metacyclogenesis. Parasitology 132:345–355
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Oakley BR, Kirsch DR, Morris R (1980) A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem 105:361–363
Duschak VG, Ciaccio M, Nasser JR, Basombrio MA (2001) Enzymatic activity, protein expression and gene sequence of cruzipain in virulent and attenuated Trypanosoma cruzi strains. J Parasitol 87:1016–1022
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Bradford J (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248
Towbin H, Staehlin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Procedures and some application. Proc Natl Acad Sci USA 76:4350–4354
Ferrero MR, Soprano LL, Acosta DM, García GA, Esteva MI, Couto AS, Duschak VG (2014) Effects of chlorate on the sulfation process of Trypanosoma cruzi glycoconjugates. Implication of parasite sulfates in cellular invasion. Acta Trop 137:161–173
Duschak VG, Cazzulo JJ (1991) Subcellular localization of glutamate dehydrogenases and alanine aminotransferase in epimastigotes of Trypanosoma cruzi. FEMS Microbiol Lett 83:131–136
Klaassen CD, Boles JW (1997) Sulfation and sulfotransferases 5: the importance of 3′-phosphoadenosine 5′-phosphosulfate (PAPS) in the regulation of sulfation. FASEB J 11(6):404–418
Baeuerle PA, Huttner WB (1986) Chlorate. A potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun 141(2):870–877
Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM (1991) Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64:841–848
Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG (1999) A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13–22
Fukuda M, Hiraoka N, Akama TO, Fukuda MN (2001) Carbohydrate-modifying sulfotransferases: structure, function, and pathophysiology. J Biol Chem 276:47747–47750
Vermelho AB, de Meirelles MDN, Pereira MC, Pohlentz G, Barreto-Bergter E (1997) Heart muscle cells share common neutral glycosphingolipids with Trypanosoma cruzi. Acta Trop 64:131–143
Petry K, Nudelman E, Eisen H, Hakomori S (1988) Sulfated lipids represent common antigens on the surface of Trypanosoma cruzi and mammalian tissues. Mol Biochem Parasitol 30:113–121
Uhrig ML, Couto AS, Zingales B, Colli W, Lederkremer RM (1992) Metabolic labelling and partial characterisation of a sulfoglycolipid in Trypanosoma cruzi trypomastigotes. Carbohydr Res 231:329–334
Bernstein HB, Compans RW (1992) Sulfation of the human immunodeficiency virus envelope glycoprotein. J Virol 66:6953–6959
Van Rooijen JJ, Kamerling JP, Vliegenthart JF (1998) Sulfated di-, tri- and tetraantennary N-glycans in human Tamm-Horsfall glycoprotein. Eur J Biochem 256:471–487
Kawasaki N, Ohta M, Hyuga S, Hashimoto O, Hayakawa T (2000) Application of liquid chromatography/mass spectrometry and liquid chromatography with tandem mass spectrometry to the analysis of the site specific carbohydrate heterogeneity in erythropoietin. Anal Biochem 285:82–91
Honke K, Taniguchi N (2002) Sulfotransferases and sulfated oligosaccharides. Med Res Rev 22:637–654
Stoka V, Nycander M, Lenarcic B, Labriola C, Cazzulo JJ, Bjork I, Turk V (1995) Inhibition of cruzipain, the major cysteine proteinase of the protozoan parasite, Trypanosoma cruzi, by proteinase inhibitors of the cystatin superfamily. FEBS Lett 370:101–104
Martínez J, Henriksson J, Ridåker M, Pettersson U, Cazzulo JJ (1998) Polymorphisms of the genes encoding cruzipain, the major cysteine proteinase of Trypanosoma cruzi, in the region encoding the C-terminal domain. FEMS Microbiol Lett 159:35–39
Cazzulo JJ, Martinez JA, Parodi AJA, Wernstedt CH, Hellman U (1992) On the post-translational modifications at the C-terminal domain of the major cysteine proteinase (cruzipain) from T. cruzi. FEMS Microbiol Lett 100:411–416
Freeze HH, Mierendorf RC, Wunderlich R, Dimond RL (1984) Sulfated oligosaccharides block antibodies to Dictyostelium discoideun acid hydrolases. J Biol Chem 259(16):10641–10643
Couto AS, Soprano LL, Landoni M, Pourcelot M, Bultel L, Parente J, Ferrero M, Barbier M, Dussouy C, Esteva M, Kovensky J, Duschak VG (2012) Anionic synthetic sugars mimic the sulfated cruzipain epitope. Central role of 6-SO3-NAcGlc for immune recognition. FEBS J 279(19):3665–3679
Campetella O, Henriksson J, Aslund L, Frasch ACC, Pettersson U, Cazzulo JJ (1992) The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is encoded by multiple polymorphic tandemly organized genes located on different chromosomes. Mol Biochem Parasitol 50:225–234
McMillan SJ, Sharma RS, McKenzie EJ, Richards HE, Zhang J, Prescott A, Crocker PR (2013) Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b β2-integrin-dependent signaling. Blood 121(11):2084–2094
Campanero-Rhodes MA, Childs RA, Kiso M, Komba S, Le Narvor C, Warren J, Otto D, Crocker PR, Feizi T (2006) Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem Biophys Res Commun 344(4):1141–1146
Redelinghuys P, Antonopoulos A, Liu Y, Campanero-Rhodes MA, McKenzie E, Haslam SM, Dell A, Feizi T, Crocker PR (2011) Early murine T-lymphocyte activation is accompanied by a switch from N-Glycolyl- to N-acetyl-neuraminic acid and generation of ligands for siglec-E. J Biol Chem 286(40):34522–34532
Acknowledgments
The authors thank the Secretaría de Ciencia y Técnica, Ministerio de Salud de la Nación Argentina and Deutscher Akademischer Austausch Dienst (MinCyT-DAAD) for the Bilateral Cooperation (DA0911), the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Agencia Nacional de Promoción Científica (ANPCyT, PICT 2006-00145), for grants provided. V.G.D. is a Research Career Investigator from CONICET, Argentina. LLS thanks FONCYT fellowship; D.M.A., M.R.F. LLS and CONICET fellowships granted. TJ and AH received funding from the collaborative research center 470 and the “Vereinigung der Freunde des Tropeninstituts.” The authors also thank to Cristina Maidana, member of the Production Department, INP Dr M. Fatala Chaben, ANLIS-Malbrán.
Author information
Authors and Affiliations
Corresponding author
Additional information
Maximiliano R. Ferrero, Anja M. Heins have contributed equally to this study.
Thomas Jacobs and Vilma G. Duschak have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Ferrero, M.R., Heins, A.M., Soprano, L.L. et al. Involvement of sulfates from cruzipain, a major antigen of Trypanosoma cruzi, in the interaction with immunomodulatory molecule Siglec-E. Med Microbiol Immunol 205, 21–35 (2016). https://doi.org/10.1007/s00430-015-0421-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-015-0421-2