Abstract
Numerosity perception is a fundamental and innate cognitive function shared by both humans and many animal species. Previous research has primarily focused on exploring the spatial and functional consistency of neural activations that were associated with the processing of numerosity information. However, the inter-individual variability of brain activations of numerosity perception remains unclear. In the present study, with a large-sample functional magnetic resonance imaging (fMRI) dataset (n = 460), we aimed to localize the functional regions related to numerosity perceptions and explore the inter-individual, hemispheric, and sex differences within these brain regions. Fifteen subject-specific activated regions, including the anterior intraparietal sulcus (aIPS), posterior intraparietal sulcus (pIPS), insula, inferior frontal gyrus (IFG), inferior temporal gyrus (ITG), premotor area (PM), middle occipital gyrus (MOG) and anterior cingulate cortex (ACC), were delineated in each individual and then used to create a functional probabilistic atlas to quantify individual variability in brain activations of numerosity processing. Though the activation percentages of most regions were higher than 60%, the intersections of most regions across individuals were considerably lower, falling below 50%, indicating substantial variations in brain activations related to numerosity processing among individuals. Furthermore, significant hemispheric and sex differences in activation location, extent, and magnitude were also found in these regions. Most activated regions in the right hemisphere had larger activation volumes and activation magnitudes, and were located more lateral and anterior than their counterparts in the left hemisphere. In addition, in most of these regions, males displayed stronger activations than females. Our findings demonstrate large inter-individual, hemispheric, and sex differences in brain activations related to numerosity processing, and our probabilistic atlas can serve as a robust functional and spatial reference for mapping the numerosity-related neural networks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estimating the number of objects in a set, namely numerosity perception, is crucial in our daily lives (Burr and Ross 2008; Piazza 2010; Dehaene et al. 1999; Feigenson et al. 2004; Piazza and Izard 2009). For example, by estimating and comparing the numbers of items in different groups, we can make more beneficial decisions, e.g., choosing a shorter queue to wait in. Over the last two decades, numerosity perception has been extensively studied, and accumulating evidence suggests that it may be innate (Xu and Spelke 2000; Berger 2011; Evans and Gold 2020; Decarli et al. 2022) and shared by both humans and many animal species (Nieder and Miller 2004; Pisa and Agrillo 2009; Piffer et al. 2012; Pica et al. 2004), and is critical for the survival and reproduction of animals and for constructing abstract and complex mathematical concepts in humans (for reviews see Piazza and Izard 2009; Burr et al. 2018).
A central topic in numerosity perception is understanding the neural mechanisms that underlie the representation and processing of numerical information. In the past two decades, significant progress has been made in applying electrophysiological and fMRI approaches to characterize the neural substrates of numerosity perception. Studies has showed that a wide range of brain structures, including the intraparietal sulcus (IPS), superior parietal lobule (SPL), insula, dorsal lateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), inferior temporal gyrus (ITG), premotor area (PM), middle occipital gyrus (MOG) and anterior cingulate cortex (ACC), were related to numerosity processing. Among these regions, a specialized parietal-frontal network, including the IPS and DLPFC was found to be consistently activated during numerosity processing (Dehaene et al. 1999, 2003; Eger et al. 2003; Pinel et al. 2004; Hayashi et al. 2013; Pinheiro-Chagas et al. 2018; Ustun et al. 2021). Activations of other brain regions, such as the SPL (Otsuka et al. 2008; Shomstein 2012), insula (Menon and Uddin 2010; Ustun et al. 2021), ACC (Menon and Uddin 2010; Ustun et al. 2021), IFG (Hampshire et al. 2009; Hayashi et al. 2013; Zhang and Iwaki 2019), MOG (Li et al. 2019; Gandini et al. 2008), and ITG (Pinheiro-Chagas et al. 2018), was also reported in relation to the attentional, and cognitive control processes required for the numerosity task. For instance, SPL has been identified as one of the key regions for arithmetic calculations (Pinheiro-Chagas et al. 2018) and the MOG is involved in attention orientation and strategy selection during numerosity estimation (Gandini et al. 2008). The insula is linked to the difficulty level in numerosity comparisons (Ustun et al. 2021), and IFG is responsible for the joint coding of numerosity and time during decision-making (Hayashi et al. 2013). The ITG plays a crucial role in digit recognition and the early identification of problem difficulty during numerosity estimation (Pinheiro-Chagas et al. 2018), and the ACC is involved in executive control, working memory, and attention processes during numerosity perception (Ustun et al. 2021). Recently, several studies also revealed the involvement of the PM in estimating the numerosity of self-generated actions, and that the characteristics of this function closely resemble those associated with the estimation of sensory numerosity, suggesting a close link between the number and action (Kansaku et al. 2006; Nieder 2017). In short, a wide range of brain structures forms the neural basis of numerosity perception, which supported the emerging network view of the neural representation of numerosity (Arsalidou et al. 2018; Zhang et al. 2023). However, previous studies have primarily focused on identifying common patterns of brain activation across individuals, i.e., the spatial and functional consistency of neural activations, the inter-individual variability in brain activations associated with the numerosity processing remains unclear.
Several fMRI studies have attempted to address the inter-individual differences in numerosity processing. They often adopted the extreme-group approach that involves comparing the brain activations of a small experimental group of individuals with a specific condition (such as dyscalculia) to a control group of healthy individuals (Ustun et al. 2021; Gandini et al. 2008). For instance, one seminal study found that people with dyscalculia exhibited much stronger activation in the right ACC than the healthy controls in a numerosity task (Ustun et al. 2021), which suggested the possible link between ACC and numerosity perception. However, certain limitations still persist in these studies. First, the small sample size of these studies often makes it challenging to identify the relationship between brain regions and numerosity processing, and to generalize their findings to explain the great variations in numerosity processing among individuals. Another limitation is that these studies often restricted their analysis to the relationship between neural activity in the specific brain region and behavioral performance (Ustun et al. 2021; Gandini et al. 2008; Haist et al. 2015). However, little is known about the inter-individual variations in numerosity processing across the entire brain. Therefore, a comprehensive and quantitative description of inter-individual variability in brain activations (particularly at the regional level) during numerosity perception is still lacking.
Moreover, previous fMRI research has found evidence for the hemispheric asymmetry in brain activations during numerosity processing (Piazza et al. 2006; Hayashi et al. 2013; Leibovich et al. 2015). Brain activations of non-symbolic number estimation appears to be right lateralized (Piazza et al. 2006; Leibovich et al. 2015), especially in the right fronto-parietal cortical network (Leibovich et al. 2015). In other regions, the right hemisphere may also play a more prominent role in numerosity processing. For example, Hayashi et al. (2013) found that the right IFG was involved in the joint coding of numerosity and time during decision-making, while the left IFG was not. However, there is still a lack of systematic investigation into the functional asymmetry of numerosity processing.
In the present study, we aimed to quantify the inter-individual variability of brain activations during the processing of numerosity information using a classical numerosity comparison task (Suarez-Pellicioni et al. 2019) and a large sample dataset of 460 participants. First, we delineated the subject-specific activated regions of interest (ROIs) responsible for numerosity processing in each individual, including the anterior and posterior IPS, IFG, ITG, PM, MOG, ACC, and insula. Then, we created a functional probabilistic atlas to quantify the spatial variability of numerosity-related brain activities, which contained precise stereotaxic information on both inter-hemispheric and inter-individual differences. Specifically, we characterized the functional and spatial variabilities in brain activities using three features: peak location, volume size, and activation magnitude of the activated regions. Besides the inter-individual variability, the inter-hemispheric and sex differences of these features were also examined. Finally, a series of evaluations were carried out to test the reliability and robustness of our functional atlas.
Materials and methods
Participants
460 healthy adults (206 males) from Beijing Normal University participated in this study, who met these following inclusion criteria: 1) normal or corrected-to-normal vision; 2) no current or past psychiatric or neurologic disorders; 3) no discomfort in a confined space; 4) no metallic objects in their body. The mean age of participants was 25.89 years (standard deviation [SD] = 1.04 years; ranging from 17 to 32 years). Four subjects were excluded from further analysis due to scanner malfunction or excessive movement inside the scanner.
Numerosity localizer task
All stimuli were generated and presented using MATLAB (MathWorks, Inc., Natick, MA) and PsychToolbox 2.54 (Brainard 1997; Pelli 1997). The stimuli were displayed on a 70 × 39.4 cm screen inside the MRI bore (resolution: 1920 × 1080 pixels, refresh rate: 60 Hz), which was positioned 120 cm away from the participant’s eyes.
Since existing research has revealed a considerable overlap in the neural substrates associated with both luminance and numerosity perception (Pinel et al. 2004; Kadosh et al. 2008; Robert et al. 2023), it is crucial to isolate the neural substrates specific to numerosity perception. To achieve this, we designed a localizer task that consisted of two conditions: number comparison and luminance comparison, as shown in Fig. 1. Employing the luminance comparison task as a control condition allowed for a more isolated examination of the brain activities specifically associated with numerosity perception. In the number comparison condition, each trial started with a 500-ms fixation point (0.34 × 0.34°) at the center of the screen, followed by the first dot array (14 × 10.4°) containing cyan dots (0.17 × 0.17°), which was presented for another 500 ms. After that, another fixation point was presented for 500 ms, followed by the second dot array presenting for another 500 ms. Participants were instructed to rapidly judge which dot array contained more dots and press the corresponding button within 2000 ms. To modulate the difficulty level in the number comparison task, we employed dot ratios of 1:2, 3:4, 5:6, 7:8, and 9:10 between the two dot arrays. The number of dots in each array was chosen from a range of 6 to 12, and all dots within both arrays exhibited uniform lightness. Therefore, the individual dot array contained either 6, 7, 8, 9, 10, or 12 dots. In this task, dot arrays were pseudo-randomly generated to ensure that subjects cannot complete the task based on the convex hull or density information (See Supplementary materials for more details; Figure S1). In the luminance comparison condition, the stimuli and procedure were the same as those in the number comparison condition, except for the following changes. Two dot arrays both contained 10 dots. The lightness of the dots in each dot array varied between (67, 151, 152) and (105, 227, 228). Participants were asked to compared which dot array had a higher luminance by pressing corresponding button. Each trial lasted 4 s. The duration of the inter-trial intervals was set at 500 ms.
The fMRI study used a blocked design and contained two runs, each lasting 376 s. Each run consisted of 6 experimental blocks, with 3 blocks for number comparison condition and 3 blocks for luminance comparison condition (Fig. 1). Each block started with a 4-s instruction display informing the participant about the condition they would perform, followed by 10 experimental trials. In addition, seven 16-s fixation blocks were interleaved with the experiential blocks. The participants were instructed to complete the task as quickly and accurately as possible. Their behavioral responses and reaction times (RTs) were recorded during the fMRI scan.
fMRI data acquisition
Functional and anatomical MRI images were acquired at the Imaging Center for Brain Research, using a Siemens Trio 3 T whole-body scanner equipped with a 12-channel phased-array head coil. Functional images were obtained using a gradient-echo planar sequence (repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, in-plane resolution = 3.5 × 3.5 mm, FOV = 232 × 232, 30 continuous axial slices, slice thickness = 4.8 mm). Structural images were acquired using a 3D T1-weighted magnetized rapid acquisition gradient echo sequence (TR = 2530 ms, TE = 3.39 ms, inversion time = 1100 ms, flip angle = 7°). Foam pillows and extendable padded head restraints were used to limit head movement. Ear plugs were used to reduce scanner noise.
Image preprocessing
Functional data were processed and analyzed using the fMRI Expert Analysis Tool (FEAT) available in the Oxford Center for Functional MRI of the Brain Software Library (FSL) (Smith et al. 2004). Preprocessing consisted of motion correction, Gaussian spatial smoothing with a 6 mm full width at half maximum and high-pass temporal filtering with a 120-s cutoff. To mitigate the effects of motion artifacts, participants whose functional data exhibiting translational or rotational deviations greater than 3 mm or 3°, respectively, were excluded from further analysis. In the first-level analysis, functional data were subjected to a General Linear Model (GLM) regression, incorporating not only the time series of task conditions—namely luminance and numerosity—but also the estimated motion parameters as nuisance regressors. The design matrix for these conditions was generated by convolving a canonical hemodynamic response function (HRF), modeled as a gamma function, with a boxcar function representing the timing and duration of the respective experimental conditions. In second-level analysis, data from each participant's two runs were integrated via fixed-effects analysis. The functional images were first aligned to individual structural scans, normalized to the MNI standard template, and resampled at a 2 × 2 × 2 mm resolution. We conducted contrasts between numerosity and luminance comparisons to isolate numerosity-related neural activities. Each participant's resultant statistical map, represented as a z-score, was used to delineate regions of brain activation specific to numerosity perception.
Labeling the functional areas of numerosity
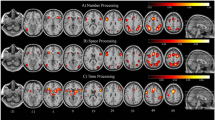
We identified eight numerosity-related regions of interest (ROIs) in both hemispheres of each participant, including the ITG, aIPS, pIPS, PM, IFG, insula, MOG, and ACC, using a semi-automated process described below (Fig. 2).
Semi-automated process used to delineate subject-specific activation regions (in one exemplar participant). A The individual activation map was derived by contrasting numerosity comparison condition vs. luminance comparison condition with z > 1.65 (p < 0.01, right-tailed, uncorrected). B The watershed algorithm was applied to divide individual activation map into small parcels and marked them with different colors. C Subject-specific ROIs were labeled based on the group-level functional reference map and anatomical landmarks
First, the activation map of each subject was thresholded at z > 1.65 (p < 0.01, right-tailed, uncorrected) (Fig. 2A). Then, we employed the Watershed algorithm to partition the activation map into small parcels (Meyer 1994) to avoid subjective determination of the boundaries between ROIs (Fig. 2B). Finally, subject-specific ROIs were selected within these parcels by two raters manually (one main rater and one assistant rater) based on the group-level functional reference map and the MNI152 template (Fig. 2C). Results of main rater were used for further analysis, while the assistant rater’s results were used to evaluate the inter-rater stability of these ROIs.
To generate the group-level functional reference map, we first averaged the binary activation map (threshold: z > 1.65) of all participants to obtain the averaged probabilistic map. To avoid any asymmetry in brain areas between hemispheres, we flipped the averaged probabilistic map, added it to the original map, and divided this result by two to obtain the final probabilistic map (Fig. 3A). Then, we applied a threshold of 0.2 to the probabilistic map and used the watershed algorithm to segment the activation areas, through which 23 clusters were obtained. Finally, four small clusters (with volume size smaller than 100 voxels) were discarded, and the remaining 19 large clusters were labeled as ROIs to acquire the group-level functional reference map (Fig. 3B). These clusters were then labeled according to the Automated Anatomical labeling (AAL3) template (Rolls et al. 2020; Wang et al. 2015) and probabilistic maps of visual topography (Rolls et al. 2020; Wang et al. 2015). Moreover, for analysis convenience, we collapsed the ACCs in both hemispheres into one cluster, labeled as bilateral ACC. The FreeROI tool was used to delineate ROIs in this procedure (Zhen et al. 2015).
Procedure used to create the group-level functional reference map. A The symmetric probabilistic map. We first averaged the binary activation map (threshold: z > 1.65) of all participants to obtain the averaged probabilistic map. Then, we flipped the averaged probabilistic map, added it to the original map, and divided this result by two to obtain the final probabilistic map. B The group-level functional reference map. We set the threshold of probabilistic map to 0.2, and labeled the corresponding large clusters as specific brain areas, including the aIPS, pIPS, insula, IFG, ITG, PM, MOG, and ACC
Functional probabilistic atlas
To quantify individual variability in the numerosity-related brain regions, a functional probabilistic atlas was constructed based on subject-specific ROIs. To do this, subject-specific ROIs were binarized and then averaged across participants to obtain the probability of activation in each region. This probability index reflected the likelihood of activation of a particular ROI at a given voxel across participants, which allowed us to obtain a voxel-based description of inter-individual variability in neural activation during a numerosity task. Finally, to construct the functional probabilistic atlas, we compared the activation probability of each ROI in each voxel. A voxel was assigned to the ROI with the maximum activation probability at that location. Any voxel with a maximum probability of less than 20% was set to 0%, as it may not belong to any ROI. By doing so, the functional probabilistic atlas for numerosity processing was created, providing non-overlapping brain map that characterized activation probability across participants. This functional probabilistic atlas will be a reliable tool for investigating inter-individual differences in neural activities.
Individual, hemispheric, and sex differences
Using these subject-specific ROIs, we can extract information about brain activation such as activation volume, magnitude, and peak value coordinates. Activation volume and peak value coordinates could be calculated directly from the ROIs in the MNI reference system. For activation magnitude, in line with previous studies (Lipkin et al. 2022; Fedorenko et al. 2010), we defined it as the percentage of signal change (PSC) of the contrast between numerosity comparison and luminance comparison conditions. In the GLM model, we obtained the estimated parameter β of the contrast of each voxel. We then divided β by the GLM intercept to obtain the PSC. The average PSC of activated voxels in a brain area was considered as the activation magnitude of that area. To assess inter-individual differences in brain activation, we calculated the mean and standard deviation of the activation volume, magnitude and peak coordinates across participants.
Next, we examined hemispheric differences and sex differences in these subject-specific ROIs. For activation volume and activation magnitude, we conducted paired two-sample t-tests to test the differences of the corresponding brain areas in different hemispheres. For peak value coordinates, we employed one-sample t-tests on the difference values of right and left areas. Since the x-axis is symmetric in right and left, the difference values were calculated by absolute values of the right areas minus absolute values of the corresponding left areas. For the y-axis and z-axis, the difference values were calculated directly from the coordinates of the right minus the coordinates of the left. Higher difference values correspond to more lateral, anterior and superior locations of the activation peak. All t-tests were corrected with FDR correction (adjusted p < 0.05).
Similarly, to explore potential sex differences in all ROIs, we measured activation volume and magnitude in male and female groups. Independent two-sample t-tests was used to examine the significance of sex differences, and multiple comparisons were corrected with FDR correction (adjusted p < 0.05).
Correlations between brain activations and behavioral performances
To prove the usability of the numerosity probabilistic atlas, we calculated the correlations between brain activation in each ROI and the behavioral performance. Behavior performance was calculated by computing the residual of accuracy and RT in a regression model where number comparison condition was the dependent variable and luminance comparison condition was the independent variable, which has been demonstrated to be effective in removing variance associated with the control condition (DeGutis et al. 2013). Activation magnitude was measured by the averaged z-score for the contrast of number comparison versus luminance comparison in each region, which can be drawn from each subject’s activation image and ROI. Among the 456 participants, 25 of them were further excluded from the correlation analysis because of their behavioral accuracies or RTs being more than three interquartile or standard deviations away from the group mean. To ensure the reliability of our results, we randomly assigned the remaining 431 subjects into two groups, one with 216 subjects and the other with 215 subjects. We only considered a brain regions’ correlation coefficient to be significant if it was found to be significant in both groups.
Evaluation of functional probabilistic atlas
Finally, we evaluated the reliability of our functional probabilistic atlas by examining four features of the atlas: anatomical correspondence (Figure S2), inter-rater reliability (Figure S3), sample size effect (Figure S4), similarity between meta-analytic maps (Figure S5) (see Supplementary Materials for details).
Results
Behavioral results
The accuracy rates were higher than 90% in both tasks, indicating that the subjects were attentive and performed the tasks accurately. Table 1 presents the behavioral results of two tasks. There was a significant difference in the accuracies between the numerosity and luminance tasks (t(430) = − 24.524, p < 0.001) (see Supplementary materials for more details).
Creating functional probabilistic atlas
During the numerosity localizer task, eight functional regions were consistently activated across individuals in each hemisphere, including the ITG, aIPS, pIPS, PM, insula, IFG, MOG, and ACC. As shown in Fig. 4, a functional probabilistic atlas of numerosity was created. The average peak coordinate of every functional region was also displayed in the atlas. Notably, the peak coordinates tended to locate in regions with higher probability.
Functional probabilistic atlas of numerosity processing (p > 0.2). The Value in each voxel represents the activation probability, which measures the likelihood that a given ROI is activated at a given voxel across participants. Any value below 0.2 was assigned a value of zero. The blue points on the figure represent the averaged peak coordinates of each numerosity-related ROI
Table 2 summarizes various measures that were used to characterize the functional probabilistic atlas, including the maximum probability and the percentage of subjects for a given ROI. The percent of subjects in each ROI ranged from 54.82% (L PM) to 95.18% (R pIPS), indicating that not all brain regions were equally involved in the numerosity comparison condition. The bilateral ITG, aIPS, pIPS, ACC, and right MOG were more consistently activated during numerosity comparison, ranging from 85.09% to 95.18%, while the bilateral PM, insula, IFG, and left MOG were less consistently activated, ranging from 54.82% to 80.48%. Another measure was the maximal activation probability of voxels within each functional region, which was found to be around 35%. This observation suggested significant individual differences in neural activation during numerosity processing. The regions with the highest probability of activation were the right pIPS (56.80%) and right ITG (52.63%), while the left PM (23.90%) and left IFG (25.88%) showed the lowest probability. These findings demonstrated the marked inter-individual variability in neural activation across various brain regions during numerosity processing.
ITG, inferior temporal gyrus, MOG middle occipital gyrus, aIPS anterior interparietal sulcus, pIPS posterior interparietal sulcus, PM premotor area, insula, IFG inferior frontal gyrus, ACC, anterior cingulate cortex, L left hemisphere, R right hemisphere BI bilateral
Inter-individual differences
The mean and standard deviation (SD) of activation volume and magnitude were shown in Table 2, while the box-plots in Fig. 5 provided more detailed information. Among these ROIs, the right pIPS exhibited the largest volume (average: 9.90 cm3), while the left MOG had the smallest volume (average: 1.88 cm3). For the activation magnitude, the right aIPS and the right pIPS showed the highest PSC (average: 0.40 for both clusters), while the right insula exhibited the lowest PSC (average: 0.25). These results demonstrated significant inter-individual differences in both activation volume and magnitude across the numerosity-related brain regions, as illustrated in Fig. 5A, B.
Distributions of activation volume and magnitude in the numerosity-related regions. A Box-plot of activation volume across individuals. B Box-plot of PSC across individuals. L, left hemisphere; R, right hemisphere; BI, bilateral. The upper and lower limits of the box represent the maximum and minimum values, respectively, and the three black dash lines within the box from top to down indicate the first quartile (25%), median (50%), and third quartile (75%). The asterisk above the short-dash line represents the significance of difference between the two hemispheres. *, p < 0.05. Multiple comparison was corrected by FDR correction (Adjusted p < 0.05)
Then, to investigate inter-individual differences in the spatial location of these brain activations, we calculated the mean and SD of the peak activation coordinates in each region along the x, y, and z axes (Table 2). The SDs in most regions were around 8 mm across participants, indicating that inter-individual differences in spatial location commonly existed in numerosity-related regions. Individual variabilities in the Y and Z axes were generally higher in the bilateral pIPS and aIPS. Figures 6A, B displayed the individual variability in peak activation coordinates, accompanied by their respective SDs.
Distributions and variability of spatial location in numerosity-related regions. (A, B) Mean coordinates and standard deviations (SD) of peak activations across individuals in the left and right hemispheres. Left–right, X axis; Anterior–posterior, Y axis; Superior-inferior, Z axis. For regions in the left hemisphere, Left–right coordinates were less than 0, and for regions in the right hemisphere, left–right coordinates were greater than 0
Hemispheric differences
Three measures were used to assess hemispheric differences in numerosity-related activation: activation volume, magnitude, and peak value coordinates.
First, several regions showed significant differences in activation volume and magnitude between the right and left hemispheres. As shown in Fig. 5A, all regions in the right hemisphere had significantly larger volumes than their counterparts in the left hemisphere (IFG: t(256) = 7.028, p < 0.001; insula: t(286) = 2.728, p = 0.007; pIPS: t(389) = 17.966, p < 0.001; ITG: t(353) = 10.350, p < 0.001; PM: t(220) = 4.807, p < 0.001; aIPS: t(372) = 13.048, p < 0.001; MOG: t(291) = 9.058, p < 0.001; after FDR correction). Similarly, most regions also showed significant hemispheric asymmetries in activation magnitudes, except for IFG (Fig. 5B). Specifically, the pIPS, ITG, PM, and aIPS exhibited stronger activation in the right hemisphere (pIPS: t(388) = 9.761, p < 0.001; ITG: t(352) = 13.914, p < 0.001; PM: t(219) = 4.639, p < 0.001; aIPS: t(370) = 17.064, p < 0.001; MOG: t(288) = 4.465, p < 0.001; after FDR correction), while the insula showed leftward asymmetry (insula: t(284) = − 4.103, p < 0.001; after FDR correction).
Furthermore, to investigate hemispheric differences in spatial locations, we calculated the coordinate differences between the right and left hemispheres and conducted one-sample t-tests on the difference of x, y, and z axes in each region. Our results were shown in Fig. 7. The majority of asymmetries were associated with the x and y axes compared to the z-axis. Specifically, for the x-axis, ITG, MOG, aIPS, pIPS, PM, and insula exhibited significant positive differences (ITG: t(353) = 8.112, p < 0.001; MOG: t(291) = 8.857, p < 0.001; aIPS: t(372) = 4.683, p < 0.001; pIPS: t(389) = 8.132, p < 0.001; PM: t(220) = 4.217, p < 0.001; insula: t(286) = 3.554, p < 0.001; after FDR correction), indicating that these regions in the right hemisphere located more laterally than their counterparts in the left hemisphere. For the y-axis, ITG, MOG, aIPS, PM, insula, and IFG showed significant positive differences (ITG: t(353) = 7.749, p < 0.001; MOG: t(291) = 4.026, p < 0.001; aIPS: t(372) = 4.240, p < 0.001; PM: t(220) = 2.334, p = 0.025; insula: t(286) = 2.893, p = 0.006; IFG: t(256) = 5.476, p < 0.001; after FDR correction), indicating that these regions were more anteriorly activated in the right than in the left hemisphere. For the z-axis, only four regions showed hemispheric differences (ITG: t(353) = − 2.767, p = 0.016; MOG: t(291) = − 2.445, p = 0.026; aIPS: t(372) = 5.978, p < 0.001; IFG: t(256) = − 2.743, p = 0.016; after FDR correction). Specifically, the right aIPS was located in a more dorsal area compared to the left, while the right ITG, MOG, and IFG were positioned more ventrally. Taken together, our results showed variability in spatial location between the two hemispheres in all regions, but this varied among the axes.
The asymmetry of coordinates in each region. Positive coordinate difference in x, y, or z axes indicated the ROI in right hemisphere located in more lateral, anterior, or dorsal position than its counterpart in the left hemisphere. Multiple comparisons were corrected with FDR correction (adjusted p < 0.05). Error bars indicate standard error of mean. ∗ , p < 0.05
Sex differences
There was no significant difference between males and females in the behavioral performance of numerosity task (t(429) = 0.040, p = 0.968). We next investigated potential sex differences in activation volume, magnitude, and peak value coordinates during numerosity processing, using an independent two-sample t-test. After correcting for multiple comparisons using the FDR correction, our results indicated no significant sex differences in peak value coordinates and activation volume. However, we found significant sex differences in activation magnitude. Specifically, males demonstrated stronger activation than females in multiple brain regions, such as left ITG, bilateral MOG, bilateral aIPS, right pIPS, bilateral insula, bilateral IFG, and the ACC, even after controlling for gray matter volume (GMV) differences between the sexes in each respective region (L ITG, t(347) = − 4.031, p = 0.001; R MOG, t(365) = − 3.799, p = 0.001; L MOG, t(293) = − 2.843, p = 0.009; R aIPS, t(389) = − 3.092, p = 0.007; L aIPS, t(368) = − 2.845, p = 0.009; R pIPS, t(410) = − 3.077, p = 0.007; R insula, t(307) = − 2.852, p = 0.009; L insula, t(295) = − 3.652, p = 0.002; R IFG, t(344) = − 2.235, p = 0.04; L IFG, t(263) = − 2.191, p = 0.04; BI ACC, t(365) = − 2.312, p = 0.04; after FDR correction) (Fig. 8) (Supplementary materials). In sum, our findings suggested that males and females exhibited similar brain activation patterns in terms of activation volume and peak value coordinates during numerosity processing, but males tended to exhibit stronger activation in certain brain regions than females.
Differences in activation magnitude between males and females in numerosity-related regions. An independent two-sample t-test was used to assess the difference in the regression residual of activation magnitude between males and females. Multiple comparisons were corrected using the FDR correction (adjusted p < 0.05). Error bars indicate standard error of mean. *, p < 0.05
Correlations between brain activations and behavioral performances
To explore the link between brain activity and behavior, we randomly divided the participants into two groups and examined the correlation between activation magnitudes in specific brain regions and the residual accuracy of the numerosity comparison task for both groups. The results showed that only the activation magnitudes in two regions, i.e., left aIPS and right pIPS, were positively correlated with the residuals of behavioral accuracy in both groups (Group 1: left aIPS: r = 0.19, p = 0.01; right pIPS: r = 0.22, p < 0.01; Group 2: left aIPS: r = 0.22, p < 0.01; right pIPS: r = 0.14, p = 0.04; Fig. 9A–D). There were also some regions showed significant correlation in only one group (Group 1: left ITG: r = 0.17, p = 0.03; right PM: r = 0.19, p = 0.03; ACC: r = 0.16, p = 0.03; Group 2: right ITG: r = 0.15, p = 0.04; left pIPS: r = 0.25, p < 0.01; left PM: r = 0.19, p = 0.04; left IFG: r = 0.18, p = 0.04). In the remaining regions, correlations were not significant in either group (all rs < 0.15, all ps > 0.05). These findings provided strong evidence for a strong association between neural activation and behavioral performance, supporting the utility of our functional probabilistic atlas. Moreover, it also suggested that our atlas could serve as a reliable and robust functional and spatial reference for standardization of functional localization in future fMRI investigations.
Correlations between brain activations and behavioral performance in left aIPS and right pIPS. The activations were calculated as the averaged z-score with the contrast of numerosity comparison versus luminance comparison. Behavioral performance of the Numerosity comparison condition was calculated as the residual of accuracy, which was obtained by regressing accuracy in the luminance comparison task from that in the numerosity comparison task. Participants were randomly divided into two groups (Group 1: N = 216; Group 2: N = 215)
Discussion
The goal of the present study is to quantify the inter-individual variability of neural activations associated with the representation and processing of numerosity information and establish a functional probabilistic atlas for numerosity-related neural processing. Using a large-sample fMRI dataset, we identified several subject-specific ROIs and these ROIs showed considerable individual, hemispheric, and sex differences in the location of peak activation, activation volume, and activation magnitude. Our functional probabilistic atlas aligned well with the meta-analytical map of numerosity created from Neurosynth (Yarkoni et al. 2011) (Supplementary materials; Fig. S5), and the correlation between activation magnitude and behavioral performance in the left aIPS and right pIPS demonstrated the practicality and feasibility of our atlas. Thus, the functional probabilistic atlas offers a robust spatial reference for the functional localization of numerosity-related neural network.
Our study diverged from earlier studies in two significant aspects. First, while most existing neuroimaging studies relied on group-averaged or anatomically defined ROIs and thus neglected individual variability, our study identified subject-specific activation areas, which allow us to systematically quantify individual differences in numerosity-related brain activities. Second, we advocated for larger sample sizes for more accurate ROI definition, addressing a frequent limitation of previous studies, as evidenced in Figure S5. Although meta-analyses have been used to mitigate the limitations imposed by small sample sizes (Cohenkadosh et al. 2008; Sokolowski et al. 2017), these approaches focused mainly on the spatial and functional consistency of neural activations across individuals, still neglecting individual variations. Therefore, our study enriched existing research by characterizing the inter-individual variability in brain activations within well-defined numerosity-related regions. These regions, including the bilateral ITG, MOG, aIPS, pIPS, PM, insula, IFG, and ACC, aligned with previously identified brain areas in the literature (Piazza et al. 2007; Piazza and Izard 2009; Holloway et al. 2010; Vogel et al. 2013; Hayashi et al. 2013; Leibovich et al. 2016; Li et al. 2019; Pinheiro-Chagas et al. 2018; Ustun et al. 2021). Such interindividual variability likely provided a neural basis for interindividual differences in numerosity perception. For instance, individual differences in functional connectivity within a distributed numerosity-related brain network could predict individual differences in the acuity of Approximate Number System (ANS) in behavior (Zhang et al. 2023).
First, in our study, we revealed substantial differences among individuals in both the functional and spatial characteristics of these numerosity-related ROIs. In general, the interindividual variability in functionally specialized cortical regions can be traced back to a combination of genetic, structural, and environmental influences during development, including variations in cytoarchitecture (e.g., Amunts et al. 1999; Caspers et al. 2013; Reardon et al. 2018), neural connectivity patterns (e.g., Passingham et al. 2002; Mueller et al. 2013; Smith et al. 2015), cortical competition (e.g., Dehaene 2013; Golarai et al. 2007; Sporns 2011; Zhao et al. 2008), and genetic factors (e.g., Hawrylycz et al. 2015). Collectively, these factors may contribute to the observed interindividual variability in brain activations related to numerosity perception. Importantly for our study, existing evidence indicated that the adolescents and adults engaged different brain regions when processing non-symbolic numerical information, and these regions undergo substantial age-related modifications (e.g., Ansari and Dhital 2006; Cantlon et al. 2006; Kaufmann et al. 2011). These findings highlighted the influence of developmental factors in shaping the neural substrates underlying non-symbolic numerosity processing, and suggested that these developmental changes could contribute to the interindividual variability we observed in numerosity-related ROIs.
Moreover, cognitive strategies employed during non-symbolic number processing might also account for the variability. Previous research has demonstrated that individuals may adopt different cognitive strategies when performing dot estimation tasks, and different cognitive strategies activated diverse brain areas. For example, Gandini et al. (2008) found that different strategies for approximate quantification during a numerosity estimation task led to the activation of distinct neural networks in young adults. Likewise, Moscoso et al. (2022) reported that numerosity estimation strategies for grouped and ungrouped stimuli engaged both common and distinct areas within the frontoparietal network. These findings, along with the sex differences elaborated upon later, may not only help reconcile disparate findings mentioned earlier in the introduction—specifically, the various brain regions previously implicated in numerosity perception—but also contribute to the observed variability in numerosity-related brain regions. Therefore, both developmental changes and strategy-based differences could be factors contributing to the interindividual variability in numerosity-related ROIs.
Interestingly, the degree of interindividual variability in brain activations differed across regions. As shown in Table 1, the bilateral IPS, particularly the right IPS, was consistently activated in more than 85% of participants and exhibited more substantial variations in activation magnitude and peak activation coordinates, in contrast to other regions which showed less consistency and smaller variations. This finding aligned with the work of Haist et al. (2015), which reported a significant developmental trajectory for improved numerosity precision across a range of brain regions, including the bilateral IPS, AG, SMG, SPL, and precuneus, as well as the left IFG and right MFG. Similarly, the right parietal cortex, especially the IPS, exhibited the most pronounced age-related effects, which may potentially account for its higher variability in activation patterns. Building on these findings, we hypothesized that the consistent activation and high variability within the IPS within the IPS may underlie its crucial role in supporting a numerical representation that is explicitly read out for numerical decisions and behavior (Eger et al. 2009; Harvey et al. 2013; Lasne et al. 2019; Piazza et al. 2004; Dormal and Pesenti 2009; Dormal et al. 2012; Lecce et al. 2015; Kersey and Cantlon 2017). Corroborating this hypothesis, we found that although all ROIs demonstrated some degree of interindividual variability, it was the variability in the activation magnitude of the right pIPS that significantly correlated with numerosity discrimination performance.
Second, we found significant hemisphere difference in these numerosity-related ROIs. For the location of peak activation and activation volume measures, regions in the right hemisphere were generally larger and located more lateral and anterior compared to their counterparts in the left hemisphere. The reason of the right asymmetry in numerosity-related regions remains largely unknown. One possibility is that, the right hemisphere mainly processes spatially non-symbolic information whereas the left hemisphere dominantly processes symbolic information (Hill et al. 2014; Braver et al. 2001). In our study, the test stimuli contained randomly distributed dots that provides non-symbolic number information as well as spatial information such as shape and location. Consistent with previous studies using similar non-symbolic stimuli (Piazza et al. 2006; Cohenkadosh et al. 2008; Dormal and Pesenti 2009; Kadosh et al. 2007), the brain activations in most regions showed right lateralization. In contrast, previous studies on symbolic numerosity, such as Arabic numbers, have shown stronger activation in the left hemisphere (Kadosh et al. 2007; Ansari 2007; Notebaert et al. 2011). For example, Notebaert et al. (2011) found that habituation and de-habituation of Arabic numbers only occurred in the left parietal cortex, indicating the left brain plays a more significant role in symbolic numerosity processing. Therefore, our findings provided new evidence, from the individual difference perspective, supporting the right-biased hemispheric asymmetry in non-symbolic numerosity processing. However, for the activation magnitude, although aIPS, pIPS, PM, MOG, and ITG showed a similar right-biased asymmetry, the insula showed a left-biased asymmetry and IFG showed no obvious asymmetry. Previous research has suggested that the accurate estimation of the numerosity in dot displays depended not only on the processing of numerosity information, but also on the ability to ignore irrelevant features interfering with the numerosity information (e.g., non-numerical magnitude properties), which may rely on inhibitory functions in the brain (Szucs et al. 2013). Thus, we speculated that the IFG and insula may be responsible for the function of execution control in numerosity processing (i.e., inhibiting the irrelevant non-numerical magnitude information), which is known to be lateralized to the left (Silva et al. 2014; John 1973).
Third, it is noteworthy that we observed significant sex differences in brain activations within these numerosity-related ROIs, even when no behavioral differences were evident between males and females. However, unlike the hemispheric differences mentioned above, sex differences were only found in the activation magnitude between males and females. Males generally had stronger activation than females in most ROIs, except for the right ITG, left pIPS, and bilateral PM. We proposed several tentative explanations for these findings. First, the behavioral tasks may not be sensitive enough to capture nuanced sex differences, whereas neuroimaging techniques like fMRI provided a more granular perspective. Second, anatomical differences in brain structure could contribute to these findings (e.g., Liu et al. 2020; Ritchie et al 2018). For instance, Keller and Menon (2009) observed sex differences in brain activity patterns during mathematical cognition tasks. They argued that these differences may be attributed to underlying anatomical differences between males and females. Females had greater regional density and volume in several posterior brain areas where functional activation differences were detected between males and females. Specifically, females showed greater density and volume in the intraparietal sulcus, parahippocampal gyrus, extrastriate cortex, supramarginal gyrus, angular gyrus, superior parietal lobule, and inferior temporal cortex. On the other hand, males did not show greater density or volume in any brain region. They concluded that the structural differences in these brain regions may contribute to the observed sex differences in brain activity patterns during mathematical tasks. However, this factor alone cannot account for our findings as we found that, after controlling for GMV variations between males and females, significant sex differences still persisted in most numerosity-related brain areas. Third, hormonal differences between males and females may also play a role in modulating cognitive function and the development of brain structure (e.g., Wallen 2009; Ritchie et al 2018). These hormones interact with neural circuits, potentially leading to sex-specific variations in brain activation patterns. For instance, Pletzer et al. (2011) revealed that sex differences in brain activations during a number bisection task were influenced by the menstrual cycle phase. Specifically, differences between males and females were more pronounced during the follicular phase and diminished during the luteal phase, particularly in regions like the medial prefrontal cortex and inferior parietal lobules. Although their findings focused on the symbolic number processing, it is reasonable to speculate that the hormonal differences might lead to the sex differences observed in the current study if considering the close relationship between symbolic and non-symbolic number representations (Carey 2011; Lipton and Spelke 2005; Dehaene 2011). Fourth, Cognitive strategies might differ between males and females, activating distinct neural circuits. These neural differences may not manifest as observable behavioral differences but could be detectable at the neural level. Although previous studies have seldom addressed the sex differences in the neural circuits underlying non-symbolic number perception, there was evidence showing that males and females recruited differential neural networks during a multi-digit number comparison task involving the symbolic numbers, and these differences were attributable to the use of different cognitive strategies by males and females (Pletzer et al. 2013). Pletzer (2016) further explored sex differences in arithmetic operations and found that males exhibited distinct neural systems for subtraction and multiplication, while these systems were largely overlapped in females, suggesting significant sex differences in brain activations associated with arithmetic operations. They argued that females may employ different neural substrates and prefer verbal and memory strategies over spatial ones compared to men to achieve similar behavioral performance, or that females might use a broader range of strategies, leading to more varied brain activation patterns. In contrast, Chen and Chang (2023) also observed sex-related patterns in the brain regions associated with arithmetic, including the left middle frontal gyrus (MFG), left intraparietal sulcus (IPS), and insula. These regions showed substantial brain responses to problem size effects in females, while males showed marginal effects. They speculated that females may rely more on algorithmic calculations and procedural strategies, while males may use faster rote-fact retrieval, estimation, and insight strategies during mathematical problem-solving. Despite of these inconsistent findings in brain activity patterns between males and females, the prevailing explanation for the sex differences has been attributed to the use of different cognitive strategies by men and women, thereby activating different brain regions (Chang et al. 2022). Given the close relationship between non-symbolic and symbolic number perception and mathematic performance (Mazzocco et al. 2011; Libertus et al. 2011; Starr et al. 2013; Tibber et al. 2012; Anobile et al. 2016, 2018), it is plausible that the observed sex differences in non-symbolic number perception could contribute to sex differences in certain high-level mathematical abilities or, at the least, these abilities may covary. Further research is needed to explore all these possibilities more thoroughly.
Finally, our study makes another significant contribution by introducing a novel functional probabilistic atlas for exploring the neural mechanisms underlying numerosity processing, which can offer several advantages. First, it may act as a quantitative spatial reference system for integrating information from multiple imaging modalities, e.g., structural, connectional, and even molecular variations in functional regions, to provide a comprehensive understanding of the neurobiological basis of numerosity processing. Second, the probabilistic atlas could facilitate fMRI research by providing subject-specific functional ROIs for group studies or as prior constraints in individual studies. At last, the probabilistic atlas can offer a more accurate reference for clinical research, allowing for the detection of any deficits in patients and quantifying deviations from healthy controls, compared to a deterministic atlas.
It should be noted that there are several potential limitations of our study. First, our participants were only college students. Thus, the validity of our findings should be confirmed in other adult populations and our findings may not be generalized to adolescents and the elderly. Future studies could include participants of different ages to examine the development of brain activity related to numerosity. Second, given that the dots in the stimuli were of uniform size, we cannot completely rule out the possibility that total surface area of the dot array may also contribute the observed brain activations. Future investigations with more refined design should be employed to eliminate this potential confound. Third, our study adopted only visual dot arrays as stimuli. To achieve a comprehensive understanding of the individual variability in supramodal numerosity processing, it's essential to include numerosity information from haptic and auditory stimuli and examine the similarities and differences between different modalities.
Conclusion
Our study thoroughly characterized the individual, hemispheric, and sex differences in brain activations during numerosity processing, offering a new perspective on inter-individual variability in the neural substrates underlying the representation and processing of numerosity information. Additionally, we developed a functional probabilistic atlas based on a large sample dataset, which can serve as a reliable functional and spatial reference for standardizing functional localization and facilitate future neuroimaging investigations.
Data availability
The probabilistic atlas that supports the findings of this study is openly available at http://www.brainactivityatlas.org. Custom code and imaging data can be accessed on GitHub at https://github.com/zangzhongyao/Data-and-code-.git. Raw data is available via a request to the corresponding author (husiyuan@bnu.edu.cn) with the need for a formal data-sharing agreement.
References
Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412(2):319–341. https://doi.org/10.1002/(sici)1096-9861(19990920)412:2%3c319::aid-cne10%3e3.0.co;2-7
Anobile G, Castaldi E, Turi M, Tinelli F, Burr DC (2016) Numerosity but not texture-density discrimination correlates with math ability in children. Dev Psychol 52(8):1206–1216. https://doi.org/10.1037/dev0000155
Anobile G, Arrighi R, Castaldi E, Grassi E, Pedonese L, Moscoso PAM, Burr DC (2018) Spatial but not temporal numerosity thresholds correlate with formal math skills in children. Dev Psychol 54(3):458–473. https://doi.org/10.1037/dev0000448
Ansari D (2007) Does the parietal cortex distinguish between “10”, “Ten”, and ten dots? Neuron 53(2):165–167. https://doi.org/10.1016/j.neuron.2007.01.001
Ansari D, Dhital B (2006) Age-related changes in the activation of the intraparietal sulcus during nonsymbolic magnitude processing: an event-related functional magnetic resonance imaging study. J Cogn Neurosci 18(11):1820–1828. https://doi.org/10.1162/jocn.2006.18.11.1820
Arsalidou M, Pawliw-Levac M, Sadeghi M, Pascual-Leone J (2018) Brain areas associated with numbers and calculations in children: meta-analyses of fMRI studies. Dev Cogn Neuros-Neth 30:239–250. https://doi.org/10.1016/j.dcn.2017.08.002
Berger A (2011) Electrophysiological evidence for numerosity processing in infancy. Dev Neuropsychol 36(6):668–681. https://doi.org/10.1080/87565641.2010.549878
Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE (2001) Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage 14(1):48–59. https://doi.org/10.1006/nimg.2001.0791
Burr DC, Anobile G, Arrighi R, (2018) Psychophysical evidence for the number sense. Philosophical Transactions of the Royal Society B: Biological Sciences 373 (1740). https://doi.org/10.1098/rstb.2017.0045
Burr D, Ross J (2008) A visual sense of number. Curr Biol 18(6):425–428. https://doi.org/10.1016/j.cub.2008.02.052
Cantlon JF, Brannon EM, Carter EJ, Pelphrey KA (2006) Functional imaging of numerical processing in adults and 4-y-old children. PLoS Biol 4(5):e125. https://doi.org/10.1371/journal.pbio.0040125
Carey S, (2011) Precis of 'The Origin of Concepts’. Behav Brain Sci 34 (3):113–124; discussion 124–162. doi:https://doi.org/10.1017/S0140525X10000919
Caspers J, Zilles K, Eickhoff SB, Schleicher A, Mohlberg H, Amunts K (2013) Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain Struct Funct 218(2):511–526. https://doi.org/10.1007/s00429-012-0411-8
Chang TT, Chen NF, Fan YT (2022) Uncovering sex/gender differences of arithmetic in the human brain: Insights from fMRI studies. Brain Behav 12(10):e2775. https://doi.org/10.1002/brb3.2775
Chen NF, Chang TT (2023) Arithmetic problem size modulates brain activations in females but not in males. Eur J Neurosci 58(5):3299–3314. https://doi.org/10.1111/ejn.16100
Cohenkadosh R, Lammertyn J, Izard V (2008) Are numbers special? an overview of chronometric, neuroimaging, developmental and comparative studies of magnitude representation. Prog Neurobiol 84(2):132–147. https://doi.org/10.1016/j.pneurobio.2007.11.001
Decarli G, Piazza M, Izard V (2022) Are infants’ preferences in the number change detection paradigm driven by sequence patterns? Infancy 28(2):206–217. https://doi.org/10.1111/infa.12505
DeGutis J, Wilmer J, Mercado RJ, Cohan S (2013) Using regression to measure holistic face processing reveals a strong link with face recognition ability. Cognition 126(1):87–100. https://doi.org/10.1016/j.cognition.2012.09.004
Dehaene S (2011) The number sense how the mind creates mathematics. Oxford University Press, New York, Rev. and updated edn
Dehaene S (2013) Inside the letterbox: how literacy transforms the human brain. Cerebrum : the Dana Forum Brain Science 2013:7
Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S (1999) Sources of mathematical thinking: behavioral and brain-imaging evidence. Science 284(5416):970–974. https://doi.org/10.1126/science.284.5416.970
Dehaene S, Piazza M, Pinel P, Cohen L (2003) Three parietal circuits for number processing. Cogn Neuropsychol 20(3):487–506. https://doi.org/10.1080/02643290244000239
Dormal V, Pesenti M (2009) Common and specific contributions of the intraparietal sulci to numerosity and length processing. Hum Brain Mapp 30(8):2466–2476. https://doi.org/10.1002/hbm.20677
Dormal V, Dormal G, Joassin F, Pesenti M (2012) A common right fronto-parietal network for numerosity and duration processing: An fMRI study. Hum Brain Mapp 33(6):1490–1501. https://doi.org/10.1002/hbm.21300
Eger E, Sterzer P, Russ MO, Giraud AL, Kleinschmidt A (2003) A supramodal number representation in human intraparietal cortex. Neuron 37(4):719–725. https://doi.org/10.1016/S0896-6273(03)00036-9
Eger E, Michel V, Thirion B, Amadon A, Dehaene S, Kleinschmidt A (2009) Deciphering cortical number coding from human brain activity patterns. Curr Biol 19(19):1608–1615. https://doi.org/10.1016/j.cub.2009.08.047
Evans LA, Gold LA (2020) Pre mathematics skills in infants: numerosity as a game. Contemp Iss Early Ch 21(1):83–86. https://doi.org/10.1177/1463949119840755
Fedorenko E, Hsieh PJ, Nieto-Castanon A, Whitfield-Gabrieli S, Kanwisher N (2010) New method for fMRI investigations of language: defining rois functionally in individual subjects. J Neurophysiol 104(2):1177–1194. https://doi.org/10.1152/jn.00032.2010
Feigenson L, Dehaene S, Spelke E (2004) Origins and endpoints of the core systems of number. Reply to Fias and Verguts. Trends Cogn Sci 8(10):448–449. https://doi.org/10.1016/j.tics.2004.08.010
Gandini D, Lemaire P, Anton JL, Nazarian B (2008) Neural correlates of approximate quantification strategies in young and older adults: An fMRI study. Brain Res 1246:144–157. https://doi.org/10.1016/j.brainres.2008.09.096
Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, Grill-Spector K (2007) Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci 10(4):512–522. https://doi.org/10.1038/nn1865
Haist F, Wazny JH, Toomarian E, Adamo M (2015) Development of brain systems for nonsymbolic numerosity and the relationship to formal math academic achievement. Hum Brain Mapp 36(2):804–826. https://doi.org/10.1002/hbm.22666
Hampshire A, Thompson R, Duncan J, Owen AM (2009) Selective tuning of the right inferior frontal gyrus during target detection. Cogn Affect Behav Ne 9(1):103–112. https://doi.org/10.3758/Cabn.9.1.103
Harvey BM, Klein BP, Petridou N, Dumoulin SO (2013) Topographic representation of numerosity in the human parietal cortex. Science 341(6150):1123–1126. https://doi.org/10.1126/science.1239052
Hawrylycz M, Miller JA, Menon V, Feng D, Dolbeare T, Guillozet-Bongaarts AL, Jegga AG, Aronow BJ, Lee CK, Bernard A, Glasser MF, Dierker DL, Menche J, Szafer A, Collman F, Grange P, Berman KA, Mihalas S, Yao Z, Stewart L, Barabasi AL, Schulkin J, Phillips J, Ng L, Dang C, Haynor DR, Jones A, Van Essen DC, Koch C, Lein E (2015) Canonical genetic signatures of the adult human brain. Nat Neurosci 18(12):1832–1844. https://doi.org/10.1038/nn.4171
Hayashi MJ, Kanai R, Tanabe HC, Yoshida Y, Carlson S, Walsh V, Sadato N (2013) Interaction of numerosity and time in prefrontal and parietal cortex. J Neurosci 33(3):883–893. https://doi.org/10.1523/Jneurosci.6257-11.2013
Hill AC, Laird AR, Robinson JL (2014) Gender differences in working memory networks: a BrainMap meta-analysis. Biol Psychol 102:18–29. https://doi.org/10.1016/j.biopsycho.2014.06.008
Holloway ID, Price GR, Ansari D (2010) Common and segregated neural pathways for the processing of symbolic and nonsymbolic numerical magnitude: An fMRI study. Neuroimage 49(1):1006–1017. https://doi.org/10.1016/j.neuroimage.2009.07.071
John K (1973) Differences between the sexes in mathematics and science courses. Int Rev Educ 19(1):47–63
Kadosh RC, Kadosh KC, Kaas A, Henik A, Goebel R (2007) Notation-dependent and -independent representations of numbers in the parietal lobes. Neuron 53(2):307–314. https://doi.org/10.1016/j.neuron.2006.12.025
Kadosh RC, Kadosh KC, Henik A (2008) When brightness counts: the neuronal correlate of numerical-luminance interference. Cereb Cortex 18(2):337–343. https://doi.org/10.1093/cercor/bhm058
Kansaku K, Johnson A, Grillon ML, Garraux G, Sadato N, Hallett M (2006) Neural correlates of counting of sequential sensory and motor events in the human brain. Neuroimage 31(2):649–660. https://doi.org/10.1016/j.neuroimage.2005.12.023
Kaufmann L, Wood G, Rubinsten O, Henik A (2011) Meta-analyses of developmental fMRI studies investigating typical and atypical trajectories of number processing and calculation. Dev Neuropsychol 36(6):763–787. https://doi.org/10.1080/87565641.2010.549884
Keller K, Menon V (2009) Gender differences in the functional and structural neuroanatomy of mathematical cognition. Neuroimage 47(1):342–352. https://doi.org/10.1016/j.neuroimage.2009.04.042
Kersey AJ, Cantlon JF (2017) Neural tuning to numerosity relates to perceptual tuning in 3–6-year-old children. J Neurosci 37(3):512–522. https://doi.org/10.1523/Jneurosci.0065-16.2017
Lasne G, Piazza M, Dehaene S, Kleinschmidt A, Eger E (2019) Discriminability of numerosity-evoked fMRI activity patterns in human intra-parietal cortex reflects behavioral numerical acuity. Cortex 114:90–101. https://doi.org/10.1016/j.cortex.2018.03.008
Lecce F, Walsh V, Didino D, Cappelletti M (2015) ‘How many’ and ‘how much’ dissociate in the parietal lobe. Cortex 73:73–79. https://doi.org/10.1016/j.cortex.2015.08.007
Leibovich T, Henik A, Salti M (2015) Numerosity processing is context driven even in the subitizing range: An fMRI study. Neuropsychologia 77:137–147. https://doi.org/10.1016/j.neuropsychologia.2015.08.016
Leibovich T, Vogel SE, Henik A, Ansari D (2016) Asymmetric processing of numerical and nonnumerical magnitudes in the brain: an fMRI study. J Cognitive Neurosci 28(1):166–176. https://doi.org/10.1162/jocn_a_00887
Li Y, Kong F, Ji M, Luo YM, Lan JJ, You XQ, (2019) Shared and Distinct Neural Bases of Large- and Small-Scale Spatial Ability: A Coordinate-Based Activation Likelihood Estimation Meta-Analysis. Front Neurosci-Switz 12. ARTN 102110.3389/fnins.2018.01021
Libertus ME, Feigenson L, Halberda J (2011) Preschool acuity of the approximate number system correlates with school math ability. Developmental Sci 14(6):1292–1300. https://doi.org/10.1111/j.1467-7687.2011.01080.x
Lipkin B, Tuckute G, Affourtit J, Small H, Mineroff Z, Kean H, Jouravlev O, Rakocevic L, Pritchett B, Siegelman M, Hoeflin C, Pongos A, Blank IA, Struhl MK, Ivanova A, Shannon S, Sathe A, Hoffmann M, Nieto-Castanon A, Fedorenko E (2022) Probabilistic atlas for the language network based on precision fMRI data from > 800 individuals. Sci Data 9 (1). doi:ARTN 52910.1038/s41597–022–01645–3
Lipton JS, Spelke ES (2005) Preschool children s mapping of number words to nonsymbolic numerosities. Child Dev 76(5):978–988. https://doi.org/10.1111/j.1467-8624.2005.00891.x
Liu S, Seidlitz J, Blumenthal JD, Clasen LS, Raznahan A (2020) Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc Natl Acad Sci U S A 117(31):18788–18798. https://doi.org/10.1073/pnas.1919091117
Maldonado Moscoso PA, Greenlee MW, Anobile G, Arrighi R, Burr DC, Castaldi E (2022) Groupitizing modifies neural coding of numerosity. Hum Brain Mapp 43(3):915–928. https://doi.org/10.1002/hbm.25694
Mazzocco MMM, Feigenson L, Halberda J (2011) Preschoolers' Precision of the Approximate Number System Predicts Later School Mathematics Performance. Plos One 6 (9). doi:ARTN e2374910.1371/journal.pone.0023749
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214(5–6):655–667. https://doi.org/10.1007/s00429-010-0262-0
Meyer F (1994) Topographic distance and watershed lines. Signal Process 38(1):113–125. https://doi.org/10.1016/0165-1684(94)90060-4
Mueller S, Wang D, Fox MD, Yeo BT, Sepulcre J, Sabuncu MR, Shafee R, Lu J, Liu H (2013) Individual variability in functional connectivity architecture of the human brain. Neuron 77(3):586–595. https://doi.org/10.1016/j.neuron.2012.12.028
Nieder A, Miller EK (2004) A parieto-frontal network for visual numerical information in the monkey. P Natl Acad Sci USA 101(19):7457–7462. https://doi.org/10.1073/pnas.0402239101
Nieder A (2017) Magnitude Codes for Cross-Modal Working Memory in the Primate Frontal Association Cortex. Front Neurosci-Switz 11. ARTN 20210.3389/fnins.2017.00202
Notebaert K, Nelis S, Reynvoet B (2011) The magnitude representation of small and large symbolic numbers in the left and right hemisphere: an event-related FMRI study. J Cognitive Neurosci 23(3):622–630. https://doi.org/10.1162/jocn.2010.21445
Otsuka Y, Osaka N, Osaka M (2008) Functional asymmetry of superior parietal lobule for working memory in the elderly. NeuroReport 19(14):1355–1359. https://doi.org/10.1097/WNR.0b013e32830e000f
Passingham RE, Stephan KE, Kotter R (2002) The anatomical basis of functional localization in the cortex. Nat Rev Neurosci 3(8):606–616. https://doi.org/10.1038/nrn893
Piazza M (2010) Neurocognitive start-up tools for symbolic number representations. Trends Cogn Sci 14(12):542–551. https://doi.org/10.1016/j.tics.2010.09.008
Piazza M, Izard V (2009) How humans count: numerosity and the parietal cortex. Neuroscientist 15(3):261–273. https://doi.org/10.1177/1073858409333073
Piazza M, Izard V, Pinel P, Le Bihan D, Dehaene S (2004) Tuning curves for approximate numerosity in the human intraparietal sulcus. Neuron 44(3):547–555. https://doi.org/10.1016/j.neuron.2004.10.014
Piazza M, Mechelli A, Price CJ, Butterworth B (2006) Exact and approximate judgements of visual and auditory numerosity: An fMRI study. Brain Res 1106:177–188. https://doi.org/10.1016/j.brainres.2006.05.104
Piazza M, Pinel P, Le Bihan D, Dehaene S (2007) A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron 53(2):293–305. https://doi.org/10.1016/j.neuron.2006.11.022
Pica P, Lemer C, Izard V, Dehaene S (2004) Exact and approximate arithmetic in an Amazonian indigene group. Science 306(5695):499–503. https://doi.org/10.1126/science.1102085
Piffer L, Agrillo C, Hyde DC (2012) Small and large number discrimination in guppies. Anim Cogn 15(2):215–221. https://doi.org/10.1007/s10071-011-0447-9
Pinel P, Piazza M, Le Bihan D, Dehaene S (2004) Distributed and overlapping cerebral representations of number, size, and luminance during comparative judgments. Neuron 41(6):983–993. https://doi.org/10.1016/S0896-6273(04)00107-2
Pinheiro-Chagas P, Daitch A, Parvizi J, Dehaene S (2018) Brain mechanisms of arithmetic: a crucial role for ventral temporal cortex. J Cognitive Neurosci 30(12):1757–1772. https://doi.org/10.1162/jocn_a_01319
Pisa P, Agrillo C (2009) Quantity discrimination in felines: a preliminary investigation of the domestic cat (Felis silvestris catus). J Ethol 27(2):289–293. https://doi.org/10.1007/s10164-008-0121-0
Pletzer B (2016) Sex differences in number processing: Differential systems for subtraction and multiplication were confirmed in men, but not in women. Sci Rep. https://doi.org/10.1038/srep39064
Pletzer B, Kronbichler M, Ladurner G, Nuerk HC, Kerschbaum H (2011) Menstrual cycle variations in the BOLD-response to a number bisection task: Implications for research on sex differences. Brain Res 1420:37–47. https://doi.org/10.1016/j.brainres.2011.08.058
Pletzer B, Kronbichler M, Nuerk HC, Kerschbaum H, (2013) Sex differences in the processing of global vs. local stimulus aspects in a two-digit number comparison task--an fMRI study. Plos One 8 (1): e53824. https://doi.org/10.1371/journal.pone.0053824
Reardon PK, Seidlitz J, Vandekar S, Liu S, Patel R, Park MTM, Alexander-Bloch A, Clasen LS, Blumenthal JD, Lalonde FM, Giedd JN, Gur RC, Gur RE, Lerch JP, Chakravarty MM, Satterthwaite TD, Shinohara RT, Raznahan A (2018) Normative brain size variation and brain shape diversity in humans. Science 360(6394):1222–1227. https://doi.org/10.1126/science.aar2578
Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E, Liewald DCM, Auyeung B, Whalley HC, Lawrie SM, Gale CR, Bastin ME, McIntosh AM, Deary IJ (2018) Sex differences in the adult human brain: evidence from 5216 UK biobank participants. Cereb Cortex 28(8):2959–2975. https://doi.org/10.1093/cercor/bhy109
Robert S, Ungerleider LG, Vaziri-Pashkam M (2023) Disentangling object category representations driven by dynamic and static visual input. J Neurosci 43(4):621–634. https://doi.org/10.1523/JNEUROSCI.0371-22.2022
Rolls ET, Huang CC, Lin CP, Feng JF, Joliot M (2020) Automated anatomical labelling atlas 3. Neuroimage 206. doi:ARTN 11618910.1016/j.neuroimage.2019.116189
Shomstein S (2012) Cognitive functions of the posterior parietal cortex: top-down and bottom-up attentional control. Front Integr Neurosci 6:38. https://doi.org/10.3389/fnint.2012.00038
Silva FPD, de Freitas SMSF, Silva PV, Banjai RM, Alouche SR (2014) Ipsilesional arm motor sequence performance after right and left hemisphere damage. J Motor Behav 46(6):407–414. https://doi.org/10.1080/00222895.2014.924473
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang YY, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. https://doi.org/10.1016/j.neuroimage.2004.07.051
Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF, Ugurbil K, Barch DM, Van Essen DC, Miller KL (2015) A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci 18(11):1565–1567. https://doi.org/10.1038/nn.4125
Sokolowski HM, Fias W, Bosah Ononye C, Ansari D (2017) Are numbers grounded in a general magnitude processing system? a functional neuroimaging meta-analysis. Neuropsychologia 105:50–69. https://doi.org/10.1016/j.neuropsychologia.2017.01.019
Sporns O (2011) The non-random brain: efficiency, economy, and complex dynamics. Front Comput Neurosci 5:5. https://doi.org/10.3389/fncom.2011.00005
Starr A, Libertus ME, Brannon EM (2013) Number sense in infancy predicts mathematical abilities in childhood. P Natl Acad Sci USA 110(45):18116–18120. https://doi.org/10.1073/pnas.1302751110
Suarez-Pellicioni M, Lytle M, Younger JW, Booth JR, (2019) A longitudinal neuroimaging dataset on arithmetic processing in school children. Sci Data ARTN 19004010.1038/sdata.2019.40
Szucs D, Nobes A, Devine A, Gabriel FC, Gebuis T, (2013) Visual stimulus parameters seriously compromise the measurement of approximate number system acuity and comparative effects between adults and children. Front Psychol ARTN 44410.3389/fpsyg.2013.00444
Tibber MS, Manasseh GS, Clarke RC, Gagin G, Swanbeck SN, Butterworth B, Lotto RB, Dakin SC (2012) Sensitivity to numerosity is not a unique visual psychophysical predictor of mathematical ability. Perception 41:228–229
Ustun S, Ayyildiz N, Kale EH, Calisir OM, Uran P, Oner O, Olkun S, Cicek M, (2021) Children With Dyscalculia Show Hippocampal Hyperactivity During Symbolic Number Perception. Front Hum Neurosci. ARTN 68747610.3389/fnhum.2021.687476
Vogel SE, Grabner RH, Schneider M, Siegler RS, Ansari D (2013) Overlapping and distinct brain regions involved in estimating the spatial position of numerical and non-numerical magnitudes: an fMRI study. Neuropsychologia 51(5):979–989. https://doi.org/10.1016/j.neuropsychologia.2013.02.001
Wallen K (2009) The Organizational hypothesis: reflections on the 50th anniversary of the publication of Phoenix, Goy, Gerall, and Young (1959). Horm Behav 55(5):561–565. https://doi.org/10.1016/j.yhbeh.2009.03.009
Wang L, Mruczek REB, Arcaro MJ, Kastner S (2015) Probabilistic maps of visual topography in human cortex. Cereb Cortex 25(10):3911–3931. https://doi.org/10.1093/cercor/bhu277
Xu F, Spelke ES (2000) Large number discrimination in 6-month-old infants. Cognition 74(1):B1–B11. https://doi.org/10.1016/s0010-0277(99)00066-9
Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011) Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8(8):665–670. https://doi.org/10.1038/nmeth.1635
Zhang D, Zhou L, Yang A, Li S, Chang C, Liu J, Zhou K (2023) A connectome-based neuromarker of nonverbal number acuity and arithmetic skills. Cereb Cortex 33(3):881–894. https://doi.org/10.1093/cercor/bhac108
Zhang F, Iwaki S (2019) Common Neural Network for Different Functions: An Investigation of Proactive and Reactive Inhibition. Front Behav Neurosci 13. doi:ARTN 124
Zhao J, Shu H, Zhang L, Wang X, Gong Q, Li P (2008) Cortical competition during language discrimination. Neuroimage 43(3):624–633. https://doi.org/10.1016/j.neuroimage.2008.07.025
Zhen ZL, Yang ZT, Huang LJ, Kong XZ, Wang X, Dang XB, Huang YY, Song YY, Liu J (2015) Quantifying interindividual variability and asymmetry of face-selective regions: a probabilistic functional atlas. Neuroimage 113:13–25. https://doi.org/10.1016/j.neuroimage.2015.03.010
Funding
This work was supported by the STI2030-Major Projects (2021ZD0203803), National Key R&D Program of China (2019YFA0709503), National Nature Science Foundation of China grant (32271108), and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
Conceptualization, KZ, SH, and JL; methodology, ZZ, and XC; investigation, ZZ, and XC; writing–original draft, ZZ; writing–review and editing, KZ and SH; funding acquisition, KZ; supervision, KZ, SH, and JL.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Ethical approval and consent to participants
All participants provided written informed consent, which was in accordance with protocol approved by the Institutional Review Board of Beijing Normal University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zang, Z., Chi, X., Luan, M. et al. Inter-individual, hemispheric and sex variability of brain activations during numerosity processing. Brain Struct Funct 229, 459–475 (2024). https://doi.org/10.1007/s00429-023-02747-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-023-02747-3