Abstract
A growing number of evidence supports a continued distribution of autistic traits in the general population. However, brain maturation trajectories of autistic traits as well as the influence of sex on these trajectories remain largely unknown. We investigated the association of autistic traits in the general population, with longitudinal gray matter (GM) maturation trajectories during the critical period of adolescence. We assessed 709 community-based adolescents (54.7% women) at age 14 and 22. After testing the effect of sex, we used whole-brain voxel-based morphometry to measure longitudinal GM volumes changes associated with autistic traits measured by the Social Responsiveness Scale (SRS) total and sub-scores. In women, we observed that the SRS was associated with slower GM volume decrease globally and in the left parahippocampus and middle temporal gyrus. The social communication sub-score correlated with slower GM volume decrease in the left parahippocampal, superior temporal gyrus, and pallidum; and the social cognition sub-score correlated with slower GM volume decrease in the left middle temporal gyrus, the right ventromedial prefrontal and orbitofrontal cortex. No longitudinal association was found in men. Autistic traits in young women were found to be associated with specific brain trajectories in regions of the social brain and the reward circuit known to be involved in Autism Spectrum Disorder. These findings support both the hypothesis of an earlier GM maturation associated with autistic traits in adolescence and of protective mechanisms in women. They advocate for further studies on brain trajectories associated with autistic traits in women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although international classifications still distinguish Autism Spectrum Disorder (ASD) on a categorical basis, a growing number of evidence supports a continued distribution of autistic traits in the general population (Whitehouse et al. 2011). Autistic traits have been reported to be associated with greater psychosocial difficulties and poorer mental health (Lai et al. 2014) and seem to share genetic vulnerability factor with ASD, with high prevalence of these traits in family members of people with ASD (Ronald and Hoekstra 2011; Robinson et al. 2011a, b; Bralten et al. 2018). However, cerebral bases of autistic traits remain largely unknown.
In people with ASD, numerous evidence has shown structural volumetric abnormalities in the frontal and temporal cortex, notably in the superior temporal sulcus, the fusiform gyrus and the insula (Boddaertet al. 2004; McAlonan et al. 2005; Hadjikhani et al. 2006; Brun et al. 2009; Greimel et al. 2013; Ecker et al. 2012; Ecker et al. 2013; Ecker et al. 2015). A relatively recent mega-analysis from the ENIGMA ASD Working Group reported that participants with ASD had significant smaller volumes of the putamen, the pallidum, the amygdala and the nucleus accumbens. In addition, they reported higher cortical thickness in the middle and superior frontal, orbitofrontal, inferior frontal, and posterior cingulate areas and lower cortical thickness in the temporal, entorhinal, and parahippocampal areas (van Rooij et al. 2018). Another recent meta-analysis revealed thicker cortical thickness in the right superior temporal, left middle temporal, anterior cingulate/paracingulate gyri, and right superior frontal (medial orbital part) gyri and higher cortical thinning the left parahippocampal gyrus, the right precentral gyrus and the left middle frontal gyrus (Shen et al. 2022).
Taken together, although controversial (Schumann et al. 2010a, b; Aylward et al. 1999; Groen et al. 2010; Hardan et al. 2001; Herbert et al. 2003; Bellani et al. 2013; Barnea-Goraly et al. 2014; Brambilla et al. 2003; Nickl-Jockschat et al. 2012; Rojas et al. 2006), these results suggest abnormalities in regions of the social brain (orbitofrontal cortex, temporal cortex, amygdala, fusiform gyrus) and the reward circuit (fronto-striatal). These characteristics may differ between sexes (Bloss and Courchesnes 2007; Craig et al. 2007; Schaer et al. 2015; Retico et al. 2016; Ecker et al. 2017; Walsh et al. 2021) with generally lower cortical thickness or volume associated with ASD in females and greater cortical thickness or volume in males (Walsh et al. 2021). However, because of the sex ratio in favor of men (Loomes et al. 2017), very few studies have included women or were specific to women.
Some other findings suggest that ASD is not associated with fixed brain abnormalities but rather support the hypothesis of a complex dynamic disorder with abnormal neuro-developmental trajectories of the brain. Several studies reported a whole brain volume increase at a faster rate during childhood, such that volume peaked in early adolescence followed by a higher decrease during early adolescence to meet the typical development curve between 10 and 15 years of age (Lange et al. 2015; Sussman et al. 2015; Mak-Fan et al. 2013; Stigler et al. 2011; Courchesne et al. 2001). Regarding cortical thickness, ASD seems to be also associated with an abnormal development with a more important thickening during early childhood, followed by an earlier thinning in later childhood leading to a decelerated thinning in early adulthood (Sussman et al. 2015; Doyle-Thomas et al. 2013; Raznahan et al. 2010; Zielinski et al. 2014), yet with important region specificities (Sussman et al. 2015; Doyle-Thomas et al. 2013; Raznahan et al. 2010; Zielinski et al. 2014). Again, distinct neurodevelopmental patterns seem to differentiate females from males with ASD (Walsh et al. 2021).
Regarding autistic traits, few studies have suggested a dimensional continuity in brain specificities along the spectrum. Using the Social Responsiveness Scale (SRS) (Constantino and Gruber 2005), in 6–10-year-old children, Blanken et al. (2015) observed a decreased gyrification in a large cluster involving the left hemisphere temporal and precuneus regions associated with autistic traits. Using the same questionnaire an association between autistic traits and cortical thickness was also found in the right superior temporal gyrus and the insula in 26 typically developing male adolescents (Tu et al. 2016). Regarding gray matter (GM) volume, results showed that autistic traits were associated with smaller volumes in the left posterior cingulate in young adults (Geurts et al. 2013). Using the Autism-Spectrum Quotient, a voxel-based morphometry (VBM) study revealed smaller GM volume in the insula associated with the 'prosocial’ dimension in males only (Saito et al. 2014). Another VBM study revealed higher GM volume in the orbitofrontal cortex, the cuneus, the hippocampus and parahippocampus and smaller GM volume in the inferior temporal cortex, the insula, and the amygdala in young men and women with high autistic traits measured by the Empathizing Quotient and the Systemizing Quotient (Focquaert and Vanneste 2015). A recent study from the IMAGEN consortium used a region of interest design to show an association between SRS scores and volumetric changes in the amygdala, the cerebellum, and the hippocampus in male and female adolescents, and in the putamen specifically in boys (Nees et al. 2022). Interestingly, brain correlates of autistic traits might also differ between sex, as suggested by a recent twin study conducted in 74 same-sex twin pairs (31 female pairs, 49 monozygotic) (Cauvet et al. 2019). Within female twin pairs increases in autistic traits (measured by the SRS) were associated with smaller cortical volume and surface area in numerous temporal and frontal regions linked to social communication. In contrast, autistic traits related less extensively with cortical volume and surface area in male twin pairs. associated with autistic traits in boys were much less extensive.
Finally, only one study investigated longitudinal brain changes associated with autistic traits. In 56 males (age 3–39 years at their first scan) followed-up for 7 years, a significant SRS score by age interaction was found in the left temporal pole and the left superior parietal cortex (Prigge et al. 2018).
Altogether, these results suggest specific brain trajectories associated with autistic traits, particularly during adolescence. However, only one study investigated longitudinal aspects, and none used a whole brain volumetric design. Moreover, sex effects of brain trajectories associated with autistic traits remain totally unexplored.
In the present neuroimaging study, we, therefore, used whole brain voxel-based morphometry to investigate whether autistic traits in the general population, measured by the SRS, are associated with longitudinal GM trajectories during adolescence (between 14 and 22 years). Due to major differences between boys and girls in terms of both prevalence of ASD and brain maturation in this critical period of adolescence (Shaw et al. 2008) the influence of sex on the age-related GM trajectories was also tested.
In addition, we aimed to have a comprehensive understanding of the distinct dimensions of autistic traits associated with GM trajectories.
Methods
Participants
Neuroimaging and clinical data were obtained from an existing large European multi-site longitudinal study of adolescent development (https://imagen-project.org) across eight European sites in France, United Kingdom, Ireland, and Germany, which includes 2,223 adolescents recruited in schools around age 14 (standard deviation = 0.41; age range: 12.9–15.7). A detailed description of recruitment and assessment procedures, with exclusion and inclusion criteria, has been published elsewhere (Schumann et al. 2010a, b). Notably, any obvious psychopathology at baseline (e.g., bipolar disorder, schizophrenia, or major neuro-developmental disorders), any severe medical somatic conditions, any history of head injuries, and any contraindications for magnetic resonance imaging (MRI) constituted exclusion criteria.
The study was approved by the ethics committees in all participating institutions. Written informed assent and consent were obtained, respectively, from all adolescents and their parents after complete description of the study.
Participants were followed up 2 years later for clinical assessment and 8 years later for clinical and neuroimaging assessment.
A total of 1182 had available MRI data at both age 14 and 22. We excluded participants who did not pass the MRI quality control, those who did not complete the SRS questionnaire and those with missing data (Fig. 1). In this study, we present data from the remaining 709 participants (n = 388 women).
MRI data
All participants underwent MRI examination on 3 Tesla scanners (General Electric, Siemens, and Philips) at 14 and 22 from the 8 European sites. High-resolution structural T1-weighted images were obtained using a standardized 3D T1-weighted magnetization prepared rapid acquisition echo (MPRAGE) sequence based on the ADNI protocol (http://adni.loni.usc.edu/methods/mri-analysis/mri-acquisition). All images passed a visual quality-control carried out by two independent raters to identify and discard images with major artifacts, such as motion, aliasing, ghosting, spikes, low signal to noise ratio. VBM was performed with the Computational Anatomy Toolbox (CAT, http://www.neuro.uni-jena.de/cat/) of Statistical Parametric Mapping 12 software (SPM12 www.fil.ion.ucl.ac.uk/spm) (Ashburner and Friston 2000).
T1-weighted images were preprocessed using a dedicated longitudinal pipeline to properly probe longitudinal (between age 14 and age 22) and cross-sectional (at both age 14 and 22) structural variations with CAT12. CAT12 provides a groupwise intra-subject modeling framework for longitudinal structural MRI data, which combines diffeomorphic and rigid-body registrations, tissue segmentation, and a correction of intensity inhomogeneity (Ashburner and Ridgway 2013). All images passed a post-preprocessing standardized CAT12 quality control. Images were retained if their quotation was D- (“sufficient”) or better corresponding to a weighted average image quality rating (IQR) > 60 (https://neuro-jena.github.io/cat12-help/).
The normalized, segmented and modulated GM images were smoothed using a 10-mm full-width at half-maximum (FWHM) Gaussian kernel. Global GM, white matter (WM), and cerebro-spinal fluid (CSF) volumes were computed for each participant at both age 14 and 22. Total Intracranial Volume (TIV) was defined as the sum of GM, WM, and CSF volumes.
Baseline characteristics
Autistic traits
Autistic traits were assessed at age 16 with the Social Responsiveness Scale (SRS), in its parent-reported form. The SRS is a well-validated questionnaire in children and adolescents aged 4 to 18 referring to five autistic dimensions: social communication, social motivation, social awareness, social cognition, restricted or repetitive behavior and interests (Constantino and Gruber 2005). Psytools software (Delosis Ltd, London, UK) was used to conduct the SRS assessment via its internet-based platform. Raw scores of SRS score and sub-scores are presented in Table 1 and were used in the analyses.
Screening for intelligence
Total Intelligence Quotient (IQ) was estimated at age 14 using the Wechsler Intelligence Scale for Children (WISC IV, Wechsler 2003).
Statistical analyses
Principal analysis
We first explored a potential sex effect in the associations between total SRS score and global GM volume variations between ages 14 and 22 within the framework of a mixed design model using R software and lme4 package. Age, SRS and sex (as assigned at birth) as main factors, and age-by-SRS-by-sex interaction were incorporated as fixed effects in the model (i.e., the following interactions: age*SRS, age*sex, SRS*sex were covered by the three-way interaction). Subject intercept and center were included as random effect. IQ and TIV were used as covariates.
If the interaction term was statistically significant, the association between total SRS score and global GM age-related changes were tested in men and women separately using a mixed design model using the same covariates.
If the age-by-SRS-by-sex triple interaction term was statistically significant, the association between total SRS score and voxelwise age-related GM changes were tested in men and women separately.
Longitudinal (between ages 14 and 22) whole brain voxelwise analyses were carried out within CAT12. To test the longitudinal effect within CAT12, we used a flexible factorial design (i.e., mixed design) with time considered as within-subject and other factors as between-subject. Age, center, IQ, TIV, and IQR were entered as confounding variables. At the voxel-level, statistical significance was set to p < 0.05 Family Wise Error (FWE) corrected for multiple comparisons. Brain locations were reported as x, y, and z coordinates in the Montreal Neurologic Institute (MNI) space.
Secondary analyses
If the previous association between total SRS score and GM volume variations was significant in the previous voxelwise analysis, we investigated the association between each SRS dimension and voxelwise GM volume variations using the same methodology.
To further complete and add a comprehensive understanding of our results, cross-sectional whole brain voxelwise analyses (at both age 14 and 22) were carried out when a significant association between SRS (total or sub-score) and voxelwise GM volume variations was observed in previous principal and secondary analyses. We used the general linear model framework provided by SPM to test multiple regression models with age, center, IQ, TIV and IQR as covariates.
Results
Clinical characteristics and global brain volumes of the 709 participants are described in Table 1.
IQR was not associated with total SRS score or with any SRS dimension.
Principal analysis
Global GM variations
A significant age-by-SRS-by-sex triple interaction term was found for global GM volume variations between ages 14 and 22 (p = 0.01). GM volume variation correlated with SRS total score in women (p value of the interaction age x SRS = 0.02, χ2 = 5.20) but not in men (p = 0.63 χ2 = 0.23) (p for age x sex interaction < 0.001) (Fig. 2). For example, in women with the lowest scores of SRS (first quintile), we observed a decrease in global GM volume of – 5.26 cm3/year, while women with the highest scores of SRS (5th quintile) demonstrated a smaller decrease of GM volume: – 3.55 cm3/year. In contrast, males demonstrated higher GM decrease that was similar in the first (– 8.42 cm3/year) and the 5th quintile (– 8.67 cm3/year). Age x sex interaction was significant in each quintile of SRS (from the first to the 5th quintile: χ2 = 26.80, p < 0.001; χ2 = 16.72, p < 0.001; χ2 = 36.31, p < 0.001; χ2 = 24.13, p < 0.001; χ2 = 64.34, p < 0.001).
Voxelwise gray matter variations
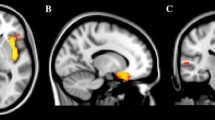
In women, the whole brain longitudinal analysis revealed that higher SRS total score correlated with slower GM volume decrease between 14 and 22 in the left parahippocampal gyrus [Brodmann area (BA) 28/34/36] and in the left middle temporal gyrus (BA 21) (Table 2, Fig. 3).
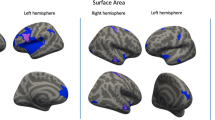
Brain regions where the Social Responsiveness Scale (SRS) total score, and the social communication and social cognition SRS sub-scores correlated with slower gray matter decrease between 14 and 22 in the 388 women. Images are presented with height threshold p < 0.001. Results are superimposed on a T1-weighted MRI scan. a Sagittal slice; b coronal slice; c transversal slice; R right. Localisation of peak-level at p < 0.05, FWE corrected in presented clusters: total SRS. a Left middle temporal gyrus, b left parahippocampus, c left parahippocampus and left middle temporal gyrus; social communication sub-score: a left superior temporal gyrus and left cerebellum, b left parahippocampus and left pallidum, c left parahippocampus, left superior temporal gyrus and left cerebellum; social cognition sub-score: a left middle temporal gyrus, b left middle temporal gyrus and left cerebellum, c left middle temporal gyrus and right ventromedial prefrontal/orbitofrontal cortex
SRS total score did not correlate with any region of GM volume variation in men.
Secondary analyses
SRS dimensions
Longitudinal whole brain analyses revealed an association between higher SRS social communication sub-scores and slower GM volume decrease in the left parahippocampal gyrus (BA 28/34/36, Fig. 4), in the left superior temporal gyrus (BA 38), the left pallidum, the left cerebellum and the left middle temporal gyrus (BA 21) (Fig. 3, Table 2) in women. In addition, SRS social cognition sub-scores correlated with slower GM volume decrease in the left middle temporal gyrus (BA 21), the right ventromedial prefrontal (vmPFC) and orbitofrontal cortex (OFC) (BA 11/25), the left cerebellum and the left superior temporal gyrus (BA 22) in women (Fig. 3, Table 2). Other SRS sub-scores (awareness, motivation and restricted or repetitive behavior and interests) did not correlate with any region of GM volume variation in women.
Cross-sectional investigations
At age 14, SRS social communication sub-score correlated with lower GM volume in the same region in women. No significant association was observed regarding total or SRS social cognition sub-score.
At age 22, no significant association was observed, neither for SRS total score nor for any of the two sub-scores.
Discussion
In the present study, we investigated for the first time the association between autistic traits in the general population and whole brain longitudinal GM volume changes during adolescence.
In women, we observed that higher autistic traits measured by the SRS were associated with slower GM volume decrease between age 14 and 22 globally and in the left parahippocampus and the left middle temporal gyrus. Left parahippocampal longitudinal variations appear to be related to social communication traits, whereas left middle temporal variations were more related to social cognition traits. In addition, social communication traits were associated with slower GM volume decrease between ages 14 and 22 in the left superior temporal gyrus and the left pallidum, and social cognition traits were associated with slower GM volume decrease in the right prefrontal ventromedial/orbitofrontal cortex. Both social communication and social cognition were associated with slower GM volume decrease in small clusters of the left cerebellum. Finally, we observed an association between social communication sub-score and smaller GM volume at age 14 in the left parahippocampus that was no longer present at age 22. No longitudinal association was found in men.
The present findings extend the very limited literature existing on brain structure development associated with autistic traits. Our results seem in line with previous findings on brain trajectories in ASD, namely, an accelerated brain maturation in childhood leading to an earlier peak-volume followed by a decelerated brain maturation in early adulthood to meet the typical development curve (Sussman et al. 2015; Doyle-Thomas et al. 2013; Raznahan et al. 2010; Zielinski et al. 2014). Indeed, we observed slower GM volume decrease between mid-adolescence and early adulthood associated with higher SRS scores. In addition, smaller GM volume in the left parahippocampus were associated with SRS social communication score at age 14 but not age 22. This could suggest that autistic traits are associated with an earlier brain maturation in regions involved in social reciprocity, and notably social communication, such as the parahippocampus. Unfortunately, we did not observe similar significant findings, of an association observed at age 14 but not 22, regarding other clusters of this study. However, on a more exploratory threshold (p < 0.001, uncorrected), we observed an association between SRS social communication sub-score and both smaller volumes in the left superior temporal gyrus and the right pallidum, that did not persist at age 22. At this more permissive threshold, smaller GM volume in several other regions were also associated with SRS scores at age 14 but not 22 (bilateral parahippocampus, left insula, left putamen for social cognition, left insula, left thalamus, bilateral parahippocampus, putamen, caudate head, bilateral fusiform, left putamen, bilateral caudate head for SRS total and social communication). Altogether, the results of the present study support the hypothesis of an earlier GM maturation associated with autistic traits in adolescence that normalizes in early adulthood. However, these results might also be seen as the result of a delay in brain development or indicative of prolonged development. Some discrepancies with the only previous report on longitudinal changes associated with autistic traits must be highlighted (Prigge et al. 2018). In the study of Prigge et al., 91 participants with ASD and 56 controls aged 3–39 years at first scan were scanned up to three times over a 7-year period to examine cortical thickness in relation to SRS score. The authors reported that SRS scores had a greater degree of age-related cortical thinning in the left temporal pole—where we found slower increase. However, the population was different from that of our study: (1) the age range was larger than in our study; (2) they included participants with ASD; (3) they studied cortical thickness and not volumes and (4) as discussed in the following paragraph, they included only males. In addition, the authors reported reduced age-related changes in the left superior parietal cortex.
Another important finding of the present study is the absence of results in male participants. Although males are predominantly represented in MRI studies on ASD, some data suggested sex-by-diagnosis effects (Craig et al. 2007; Sussman et al. 2015). Interestingly, such an effect was reported in the bilateral parahippocampal (BA 28/34), the middle temporal lobe (BA 21/22) and the vmPFC/OFC (Schaer et al. 2015; Ecker et al. 2019), that are regions in which we observed slower GM decrease in females—but not in males—associated with higher autistic traits. A recent meta-analysis (Walsh et al. 2021) confirmed and extended this sex-by-diagnosis effect within regions associated with the limbic, default mode, visual, and auditory/language regions networks. This effect was mainly explained by atypically lower volume or thickness in females with ASD compared to trends toward higher values in males with ASD, with an age-dependency of regional sex-by-diagnosis effects suggested by the authors. More generally, a growing literature supports the existence of greater extend of structural brain alterations associated with autism and autistic traits in females (Bloss and Courchesnes 2007; Schumann et al. 2009; Schumann et al. 2010a, b; Ecker, 2017; Cauvet et al. 2019; Van't Westeinde et al. 2019; Walsh et al. 2021). For example, Bloss and Courchesne observed that girls with ASD exhibited additional sites of abnormalities compared to boys with ASD, notably in temporal regions (Bloss and Courchesnes 2007). In a sub-sample of ASD-discordant pairs, Van’t Westeinde et al. found extended increased thickness in the orbitofrontal regions, superior frontal gyrus, and intraparietal sulcus associated with restricted and repetitive behaviors and interests in females, whereas males only presented increased volume in the bilateral pallidum (Van't Westeinde et al. 2019). On a developmental point of view, females with ASD were found to display a more pronounced abnormal growth profile in more brain regions than males with ASD (Schumann et al. 2010a, b). In addition, one recent twin study on adolescents and young adults reported important brain correlates associated with autistic traits in females, and relatively few correlates in males (Cauvet et al. 2019). In women, higher autistic traits, also measured by the SRS, were associated with smaller volumes and surface areas in regions similar to those observed in the present study, notably the left superior temporal gyrus, the middle temporal gyrus, and the parahippocampus. Males had different patterns with fewer regions associated with autistic traits, including, however, the parahippocampus. Evidence for functional and microstructural studies also support more pronounced brain particularities associated with ASD (Walsh et al. 2021). Altogether these results suggest that females might possess protective mechanisms regarding ASD and require a higher detrimental load, including structural brain alterations, before developing clinically relevant levels of autistic traits. Effect of sex steroids on brain development may interact with ASD risk genes to produce these sex differences (Walsh et al. 2021; Ferri et al. 2018). Altogether, these results and hypotheses advocate for further studies focusing on brain correlates and trajectories associated with autistic traits and ASD in females.
The most important region highlighted in the present study is the left parahippocampus. Lower thickness of this region has been associated with ASD in the mega-analysis of van Rooij et al. (2018) but the authors did not report any effect of age. In addition, one previous study reported volumetric particularities in the parahippocampus associated with autistic traits, but the findings were of larger volume in the right parahippocampus (Focquaert and Vanneste 2015). The parahippocampal cortex has been associated with many cognitive processes, such as episodic memory, visuospatial processing, spatial representation and navigation, center-periphery of the visual field distinction, processing of emotional stimuli and treatment of auditory and odor stimuli (Aminoff et al. 2013). Aminoff et al. (2013) suggested that the parahippocampal cortex is part of a network of brain regions that processes contextual associations underlying these different cognitive processes. Interestingly, deficits in contextual information processing is present in ASD and a poor global processing, and consequently poor context processing, characterizes the Weak Central Coherence theory proposed to describe autistics behaviors (Ben-Yosef et al. 2017).
Also, in the present study, the communication sub-score was associated with slower volumetric decrease in the left superior temporal gyrus and the left pallidum. In ASD, abnormalities of these two regions have been largely reported (van Rooij et al. 2018; Shen et al. 2022; Sussman et al. 2015; Weerasekera et al. 2022). The superior temporal gyrus (STG) is involved in auditory processing, including language, but also in social cognition (Bigler et al. 2007). Part of the basal ganglia, the pallidum is involved in several important functions in the brain, including sensory control, motor programming, and reward-driven behaviors. In ASD, however, abnormalities of the pallidum have been more associated with restricted and repetitive behaviors and interests (Estes et al. 2011; Wilkes and Lewis 2018). A recent twin study reported increased volume of the bilateral pallidum associated with restricted and repetitive behaviors and interests in males but not in females (Van’t Westeinde et al. 2019).
In the present study, two regions were particularly associated with the social cognition dimension: the left middle temporal gyrus (MTG) and the right vmPFC/OFC. The PFC is the core brain region in the mentalizing network and is considered as a central hub of the “social brain” (Han et al. 2021). The vmPFC and OFC are involved in the emotional part of the theory of mind and are mainly responsible for understanding the emotional state of others (Abu-Akel and Shamay-Tsoory 2011). Larger GM volumes in these regions were found in individuals with a larger social network size (Kanai et al. 2012) and in those with higher mentalizing competence (Powell et al. 2012). Recently, a clinical trial observed that autistic children randomized in social cognitive skills groups demonstrated increased activity in the mPFC during an irony task that was associated with an improvement in social functioning on the SRS (Ibrahim et al. 2021). The left MTG is also involved in the social brain and the theory of mind. It is a critical node of the brain’s language network, and is notably involved in communicative-pragmatics, i.e., the ability to use language to convey meaning in a specific context by requiring inferential steps (Morese et al. 2022). More specifically, left MTG has been associated with comprehension of lexical ambiguity (Acheson and Hagoort 2013) and irony (Rapp et al. 2013). Several fMRI studies of healthy participants reported an activation of the left MTG for the correct understanding of irony (Bosco et al. 2017), semantic integration (Noppeney and Price 2004) or analysis of the coherence of a text (Morese et al. 2022). Left MTG could thus play a pivotal role in the understanding of complex communication. Finally, decreased connectivity between the orbitofrontal cortex and the MTG have been reported in adults with ASD (Cheng et al. 2017).
The strengths of this study are the relatively large and homogenous adolescent/young adult population, the use of confounding variable, such as IQ for the entire sample, the 8-year follow-up and the whole brain longitudinal protocol. The most important limitations regard the use of the SRS. First, the SRS has been shown to be relatively unspecific, with high score found in individuals with other mental or neurodevelopmental disorders (e.g.,Capriola-Hall et al. 2021; Reiersen et al. 2007). Second, in the present study, parent-reported SRS scores were measured at age 16, whereas imaging data were collected at age 14 and 22. However, autistic traits are known to be stable (Robinson et al. 2011a, b) even from childhood through early adulthood (Wagner et al. 2019).
Conclusions
Overall, the present study demonstrated for the first-time specific brain trajectories, from mid-adolescence to young adulthood, associated with autistic traits in young women from the general population. These specific brain trajectories were observed in regions of the social brain and the reward circuit known to be involved in ASD. The present findings are in line with previous literature and could support the hypothesis of an earlier GM maturation associated with higher autistic traits in adolescence that normalizes in early adulthood. The absence of results in males support the hypothesis of previous studies that females might possess protective mechanisms regarding ASD and require a higher detrimental load, including structural brain alterations, before developing clinically relevant levels of autistic traits. Altogether, these results advocate for further studies focusing on brain correlates and trajectories associated with autistic traits and ASD in females.
Data availability
Imagen data can be requested here: https://imagen-project.org/the-imagen-dataset/.
References
Abu-Akel A, Shamay-Tsoory S (2011) Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia 49(11):2971–2984. https://doi.org/10.1016/j.neuropsychologia.2011.07.012
Acheson DJ, Hagoort P (2013) Stimulating the brain’s language network: syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J Cogn Neurosci 25(10):1664–1677. https://doi.org/10.1162/jocn_a_00430
Aminoff EM, Kveraga K, Bar M (2013) The role of the parahippocampal cortex in cognition. Trends Cogn Sci 17(8):379–390. https://doi.org/10.1016/j.tics.2013.06.009
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11(6 Pt 1):805–821. https://doi.org/10.1006/nimg.2000.0582
Ashburner J, Ridgway GR (2013) Symmetric diffeomorphic modeling of longitudinal structural MRI. Front Neurosci 6:197. https://doi.org/10.3389/fnins.2012.00197
Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD (1999) Mri volumes of amygdala and hippocampus in nonmentally retarded autistic adolescents and adults. Neurology 53:2145–2150. https://doi.org/10.1212/wnl.53.9.2145
Barnea-Goraly N, Frazier TW, Piacenza L, Minshew NJ, Keshavan MS, Reiss AL, Hardan AY (2014) A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog Neuro-Psychopharmacol Biol Psychiatry 48:124–128. https://doi.org/10.1016/j.pnpbp.2013.09.010
Bellani M, Calderoni S, Muratori F, Brambilla P (2013) Brain anatomy of autism spectrum disorders II. Focus on Amygdala Epidemiol Psychiatr Sci 22:309–312. https://doi.org/10.1017/S2045796013000346
Ben-Yosef D, Anaki D, Golan O (2017) Context processing in adolescents with autism spectrum disorder: how complex could it be? Autism Res 10(3):520–530. https://doi.org/10.1002/aur.1676
Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lu J, Provencal SL, McMahon W, Lainhart JE (2007) Superior temporal gyrus, language function, and autism. Dev Neuropsychol 31(2):217–238. https://doi.org/10.1080/87565640701190841
Blanken LM, Mous SE, Ghassabian A, Muetzel RL, Schoemaker NK, El Marroun H, van der Lugt A, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H, White T (2015) Cortical morphology in 6- to 10-year old children with autistic traits: a population-based neuroimaging study. Am J Psychiatry 172(5):479–486. https://doi.org/10.1176/appi.ajp.2014.14040482
Bloss CS, Courchesne E (2007) MRI neuroanatomy in young girls with autism: a preliminary study. J Am Acad Child Adolesc Psychiatry 46(4):515–523. https://doi.org/10.1097/chi.0b013e318030e28b
Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthélémy C, Mouren MC, Artiges E, Samson Y, Brunelle F, Frackowiak RS, Zilbovicius M (2004) Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage 23(1):364–369. https://doi.org/10.1016/j.neuroimage.2004.06.016
Bosco FM, Parola A, Valentini MC, Morese R (2017) Neural correlates underlying the comprehension of deceitful and ironic communicative intentions. Cortex 94:73–86. https://doi.org/10.1016/j.cortex.2017.06.010
Bralten J, van Hulzen KJ, Martens MB, Galesloot TE, Arias Vasquez A, Kiemeney LA, Buitelaar JK, Muntjewerff JW, Franke B, Poelmans G (2018) Autism spectrum disorders and autistic traits share genetics and biology. Mol Psychiatry 23(5):1205–1212. https://doi.org/10.1038/mp.2017.98
Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F (2003) Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull 61:557–569. https://doi.org/10.1016/j.brainresbull.2003.06.001
Capriola-Hall NN, McFayden T, Ollendick TH, White SW (2021) Caution when screening for autism among socially anxious youth. J Autism Dev Disord 51(5):1540–1549. https://doi.org/10.1007/s10803-020-04642-w
Cauvet É, Van’t Westeinde A, Toro R, Kuja-Halkola R, Neufeld J, Mevel K, Bölte S (2019) Sex differences along the autism continuum: a twin study of brain structure. Cereb Cortex 29(3):1342–1350. https://doi.org/10.1093/cercor/bhy303
Cheng W, Rolls ET, Zhang J, Sheng W, Ma L, Wan L, Luo Q, Feng J (2017) Functional connectivity decreases in autism in emotion, self, and face circuits identified by knowledge-based enrichment analysis. Neuroimage 148:169–178. https://doi.org/10.1016/j.neuroimage.2016.12.068
Constantino JN, Gruber CP (2005) Social responsiveness scale (SRS). Western psychological services, Los Angeles, CA
Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY (2001) Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57(2):245–254. https://doi.org/10.1212/wnl.57.2.245
Craig MC, Zaman SH, Daly EM, Cutter WJ, Robertson DM, Hallahan B, Toal F, Reed S, Ambikapathy A, Brammer M, Murphy CM, Murphy DG (2007) Women with autistic-spectrum disorder: magnetic resonance imaging study of brain anatomy. Br J Psychiatry 191:224–228. https://doi.org/10.1192/bjp.bp.106.034603
Doyle-Thomas KA, Duerden EG, Taylor MJ, Lerch JP, Soorya LV, Wang AT, Fan J, Hollander E, Anagnostou E (2013) Effects of age and symptomatology on cortical thickness in autism spectrum disorders. Res Autism Spectr Disord 7(1):141–150. https://doi.org/10.1016/j.rasd.2012.08.004
Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, Catani M, Jezzard P, Barnes A, Bailey AJ, Williams SC, Murphy DG, MRC AIMS Consortium (2012) Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry 69(2):195–209. https://doi.org/10.1001/archgenpsychiatry.2011.1251
Ecker C, Ginestet C, Feng Y, Johnston P, Lombardo MV, Lai MC, Suckling J, Palaniyappan L, Daly E, Murphy CM, Williams SC, Bullmore ET, Baron-Cohen S, Brammer M, Murphy DG, MRC AIMS Consortium (2013) Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiat 70(1):59–70. https://doi.org/10.1001/jamapsychiatry.2013.265
Ecker C, Bookheimer SY, Murphy DG (2015) Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol 14(11):1121–1134. https://doi.org/10.1016/S1474-4422(15)00050-2
Ecker C, Andrews DS, Gudbrandsen CM, Marquand AF, Ginestet CE, Daly EM, Murphy CM, Lai MC, Lombardo MV, Ruigrok AN, Bullmore ET, Suckling J, Williams SC, Baron-Cohen S, Craig MC, Murphy DG, Medical Research Council Autism Imaging Multicentre Study (MRC AIMS) Consortium (2019) Association between the probability of autism spectrum disorder and normative sex-related phenotypic diversity in brain structure. JAMA Psychiat 76(5):549–550. https://doi.org/10.1001/jamapsychiatry.2018.4296
Estes A, Shaw DW, Sparks BF, Friedman S, Giedd JN, Dawson G, Bryan M, Dager SR (2011) Basal ganglia morphometry and repetitive behavior in young children with autism spectrum disorder. Autism Res 4(3):212–220. https://doi.org/10.1002/aur.193
Ferri SL, Abel T, Brodkin ES (2018) Sex differences in autism spectrum disorder: a review. Curr Psychiatry Rep 20(2):9. https://doi.org/10.1007/s11920-018-0874-2
Focquaert F, Vanneste S (2015) Autism spectrum traits in normal individuals: a preliminary VBM analysis. Front Hum Neurosci 9:264. https://doi.org/10.3389/fnhum.2015.00264
Geurts HM, Ridderinkhof KR, Scholte HS (2013) The relationship between grey-matter and ASD and ADHD traits in typical adults. J Autism Dev Disord 43(7):1630–1641. https://doi.org/10.1007/s10803-012-1708-4
Greimel E, Nehrkorn B, Schulte-Rüther M, Fink GR, Nickl-Jockschat T, Herpertz-Dahlmann B, Konrad K, Eickhoff SB (2013) Changes in grey matter development in autism spectrum disorder. Brain Struct Funct 218(4):929–942. https://doi.org/10.1007/s00429-012-0439-9
Groen W, Teluij M, Buitelaar J, Tendolkar I (2010) Amygdala and hippocampus enlargement during adolescence in autism. J Am Acad Child Adolesc Psychiatry 49:552–560. https://doi.org/10.1016/j.jaac.2009.12.023
Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H (2006) Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex 16(9):1276–1282. https://doi.org/10.1093/cercor/bhj069
Han M, Jiang G, Luo H, Shao Y (2021) Neurobiological bases of social networks. Front Psychol 12:626337. https://doi.org/10.3389/fpsyg.2021.626337
Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS (2001) Brain volume in autism. J Child Neurol 16:421–424. https://doi.org/10.1177/088307380101600607
Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness VS Jr (2003) Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain 126:1182–1192. https://doi.org/10.1093/brain/awg110
Ibrahim K, Soorya LV, Halpern DB, Gorenstein M, Siper PM, Wang AT (2021) Social cognitive skills groups increase medial prefrontal cortex activity in children with autism spectrum disorder. Autism Res 14(12):2495–2511. https://doi.org/10.1002/aur.2603
Kanai R, Bahrami B, Roylance R, Rees G (2012) Online social network size is reflected in human brain structure. Proc Biol Sci 279(1732):1327–1334. https://doi.org/10.1098/rspb.2011.1959
Lai MC, Lombardo MV, Baron-Cohen S (2014) Autism. Lancet 383(9920):896–910. https://doi.org/10.1016/S0140-6736(13)61539-1
Lange N, Travers BG, Bigler ED, Prigge MB, Froehlich AL, Nielsen JA, Cariello AN, Zielinski BA, Anderson JS, Fletcher PT, Alexander AA, Lainhart JE (2015) Longitudinal volumetric brain changes in autism spectrum disorder ages 6–35 years. Autism Res 8(1):82–93. https://doi.org/10.1002/aur.1427
Loomes R, Hull L, Mandy WPL (2017) What is the male-to-female ratio in autism spectrum disorder? a systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 56(6):466–474. https://doi.org/10.1016/j.jaac.2017.03.013
Mak-Fan KM, Morris D, Vidal J, Anagnostou E, Roberts W, Taylor MJ (2013) White matter and development in children with an autism spectrum disorder. Autism 17(5):541–557. https://doi.org/10.1177/1362361312442596
McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, Yip L, Murphy DG, Chua SE (2005) Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain 128(Pt 2):268–276. https://doi.org/10.1093/brain/awh332
Morese R, Brasso C, Stanziano M, Parola A, Valentini MC, Bosco FM, Rocca P (2022) Efforts for the correct comprehension of deceitful and ironic communicative intentions in schizophrenia: a functional magnetic resonance imaging study on the role of the left middle temporal gyrus. Front Psychol 13:866160. https://doi.org/10.3389/fpsyg.2022.866160
Nees F, Banaschewski T, Bokde ALW, Desrivières S, Grigis A, Garavan H, Gowland P, Grimmer Y, Heinz A, Brühl R, Isensee C, Becker A, Martinot JL, Paillère Martinot ML, Artiges E, Papadopoulos Orfanos D, Lemaître H, Stringaris A, van Noort B, On Behalf of The Imagen Consortium et al (2022) Global and regional structural differences and prediction of autistic traits during adolescence. Brain Sci 12(9):1187. https://doi.org/10.3390/brainsci12091187
Nickl-Jockschat T, Habel U, Michel TM, Manning J, Laird AR, Fox PT, Schneider F, Eickhoff SB (2012) Brain structure anomalies in autism spectrum disorder—a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp 33:1470–1489. https://doi.org/10.1002/hbm.21299
Noppeney U, Price CJ (2004) An FMRI study of syntactic adaptation. J Cogn Neurosci 16(4):702–713. https://doi.org/10.1162/089892904323057399
Powell J, Lewis PA, Roberts N, García-Fiñana M, Dunbar RI (2012) Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proc Biol Sci 279(1736):2157–2162. https://doi.org/10.1098/rspb.2011.2574
Prigge MBD, Bigler ED, Travers BG, Froehlich A, Abildskov T, Anderson JS, Alexander AL, Lange N, Lainhart JE, Zielinski BA (2018) Social responsiveness scale (SRS) in relation to longitudinal cortical thickness changes in autism spectrum disorder. J Autism Dev Disord 48(10):3319–3329. https://doi.org/10.1007/s10803-018-3566-1
Rapp AM, Langohr K, Mutschler DE, Klingberg S, Wild B, Erb M (2013) Isn’t it ironic? neural correlates of irony comprehension in schizophrenia. PLoS ONE 8(9):e74224. https://doi.org/10.1371/journal.pone.0074224
Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, Bolton PF, Paus T, Murphy DG (2010) Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex 20(6):1332–1340. https://doi.org/10.1093/cercor/bhp198
Reiersen AM, Constantino JN, Volk HE, Todd RD (2007) Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry 48(5):464–472. https://doi.org/10.1111/j.1469-7610.2006.01720.x
Retico A, Giuliano A, Tancredi R, Cosenza A, Apicella F, Narzisi A, Biagi L, Tosetti M, Muratori F, Calderoni S (2016) The effect of gender on the neuroanatomy of children with autism spectrum disorders: a support vector machine case-control study. Mol Autism 7:5. https://doi.org/10.1186/s13229-015-0067-3
Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happé F, Plomin R, Ronald A (2011a) Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%). Arch Gen Psychiatry 68(11):1113–1121. https://doi.org/10.1001/archgenpsychiatry.2011.119
Robinson EB, Munir K, Munafò MR, Hughes M, McCormick MC, Koenen KC (2011b) Stability of autistic traits in the general population: further evidence for a continuum of impairment. J Am Acad Child Adolesc Psychiatry 50(4):376–384. https://doi.org/10.1016/j.jaac.2011.01.005
Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR (2006) Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 6:56. https://doi.org/10.1186/1471-244X-6-56
Ronald A, Hoekstra RA (2011) Autism spectrum disorders and autistic traits: a decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet 156B(3):255–274. https://doi.org/10.1002/ajmg.b.31159
Saito Y, Suga M, Tochigi M, Abe O, Yahata N, Kawakubo Y, Liu X, Kawamura Y, Sasaki T, Kasai K, Yamasue H (2014) Neural correlate of autistic-like traits and a common allele in the oxytocin receptor gene. Soc Cogn Affect Neurosci 9(10):1443–1450. https://doi.org/10.1093/scan/nst136
Schaer M, Kochalka J, Padmanabhan A, Supekar K, Menon V (2015) Sex differences in cortical volume and gyrification in autism. Mol Autism 6:42. https://doi.org/10.1186/s13229-015-0035-y
Schumann CM, Barnes CC, Lord C, Courchesne E (2009) Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry 66(10):942–949. https://doi.org/10.1016/j.biopsych.2009.07.007
Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Pierce K, Hagler D, Schork N, Lord C, Courchesne E (2010a) Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci 30:4419–4427. https://doi.org/10.1523/JNEUROSCI.5714-09.2010
Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, Conrod PJ, Dalley JW, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Mallik C, Mann K, Martinot JL, Paus T, Poline JB, IMAGEN consortium et al (2010b) The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry 15(12):1128–1139. https://doi.org/10.1038/mp.2010.4
Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP (2008) Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28(14):3586–3594. https://doi.org/10.1523/JNEUROSCI.5309-07.2008
Shen L, Zhang J, Fan S, Ping L, Yu H, Xu F, Cheng Y, Xu X, Yang C, Zhou C (2022) Cortical thickness abnormalities in autism spectrum disorder. Eur Child Adolesc Psychiatry. https://doi.org/10.1007/s00787-022-02133-0
Stigler KA, McDonald BC, Anand A, Saykin AJ, McDougle CJ (2011) Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Res 1380:146–161. https://doi.org/10.1016/j.brainres.2010.11.076
Sussman D, Leung RC, Vogan VM, Lee W, Trelle S, Lin S, Cassel DB, Chakravarty MM, Lerch JP, Anagnostou E, Taylor MJ (2015) The autism puzzle: diffuse but not pervasive neuroanatomical abnormalities in children with ASD. Neuroimage Clin 8:170–179. https://doi.org/10.1016/j.nicl.2015.04.008
Tu PC, Hsu JW, Lan CC, Liu CC, Su TP, Chen YS (2016) Structural and functional correlates of a quantitative autistic trait measured using the social responsive scale in neurotypical male adolescents. Autism Res 9(5):570–578. https://doi.org/10.1002/aur.1535
van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, Calderoni S, Daly E, Deruelle C, Di Martino A, Dinstein I, Duran FLS, Durston S, Ecker C, Fair D, Fedor J, Fitzgerald J, Freitag CM, Gallagher L et al (2018) Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the enigma asd working group. Am J Psychiatry 175(4):359–369. https://doi.org/10.1176/appi.ajp.2017.17010100
Van’t Westeinde A, Cauvet É, Toro R, Kuja-Halkola R, Neufeld J, Mevel K, Bölte S (2019) Sex differences in brain structure: a twin study on restricted and repetitive behaviors in twin pairs with and without autism. Mol Autism 11(1):1. https://doi.org/10.1186/s13229-019-0309-x
Wagner RE, Zhang Y, Gray T, Abbacchi A, Cormier D, Todorov A, Constantino JN (2019) Autism-related variation in reciprocal social behavior: a longitudinal study. Child Dev 90(2):441–451. https://doi.org/10.1111/cdev.13170
Walsh MJM, Wallace GL, Gallegos SM, Braden BB (2021) Brain-based sex differences in autism spectrum disorder across the lifespan: a systematic review of structural MRI, fMRI, and DTI findings. Neuroimage Clin. 31:102719. https://doi.org/10.1016/j.nicl.2021.102719
Wechsler D (2003) Wechsler intelligence scale for children, 4th edn. PsychCorp, San Antonia, TX
Weerasekera A, Ion-Mărgineanu A, Nolan G, Mody M (2022) Subcortical brain morphometry differences between adults with autism spectrum disorder and schizophrenia. Brain Sci 12(4):439. https://doi.org/10.3390/brainsci12040439
Whitehouse AJ, Hickey M, Ronald A (2011) Are autistic traits in the general population stable across development? PLoS ONE 6(8):e23029. https://doi.org/10.1371/journal.pone.0023029
Wilkes BJ, Lewis MH (2018) The neural circuitry of restricted repetitive behavior: magnetic resonance imaging in neurodevelopmental disorders and animal models. Neurosci Biobehav Rev 92:152–171. https://doi.org/10.1016/j.neubiorev.2018.05.022
Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, Fletcher PT, Zygmunt KM, Travers BG, Lange N, Alexander AL, Bigler ED, Lainhart JE (2014) Longitudinal changes in cortical thickness in autism and typical development. Brain 137(Pt 6):1799–1812. https://doi.org/10.1093/brain/awu083
Acknowledgements
Imagen Consortium collaborators (https://imagen-project.org/): Eric Artiges, Semiha Aydin, Christine Bach, Tobias Banaschewski, Alexis Barbot, Gareth Barker, Arun Bokde, Nadège Bordas, Zuleima Bricaud, Uli Bromberg, Ruediger Bruehl, Christian Büchel, Anna Cattrell, Patricia Conrod, Sylvane Desrivieres, Tahmine Fadai, Irina Filippi, Herta Flor, Vincent Frouin, André Galinowski, Jürgen Gallinat, Hugh Garavan, Fanny Gollier-Briant, Chantal Gourlan, Penny Gowland, Stella Guldner, Andreas Heinz, Bernd Ittermann, Tianye Jia, Hervé Lemaitre, Jean-Luc Martinot, Jessica Massicotte, Ruben Miranda, Kathrin Müller, Frauke Nees, Charlotte Nymberg, Marie Laure Paillère Martinot, Tomas Paus, Zdenka Pausova, Jean-Baptiste Poline, Luise Poustka, Jan Reuter, John Rogers, Barbara Ruggeri, Anna S Sarvasmaa, Christine Schmäl, Gunter Schumann, Maren Struve, Michael Smolka, Wolfgang Sommer, Hélène Vulser, Henrik Walter, Robert Whelan.
Funding
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT-2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network-based stratification of reinforcement-related disorders) (695313), Human Brain Project (HBP SGA 2, 785907, and HBP SGA 3, 945539), the Medical Research Council Grant ‘c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the National Institute of Health (NIH) (R01DA049238, A decentralized macro and micro gene-by-environment interaction analysis of substance use behavior and its brain biomarkers), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; Forschungsnetz AERIAL 01EE1406A, 01EE1406B; Forschungsnetz IMAC-Mind 01GL1745B), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-2, SFB 940, TRR 265, NE 1383/14-1), the Medical Research Foundation and Medical Research Council (grants MR/R00465X/1 and MR/S020306/1), the National Institutes of Health (NIH) funded ENIGMA (grants 5U54EB020403-05 and 1R56AG058854-01), NSFC grant 82150710554 and European Union funded project ‘environMENTAL’, grant no: 101057429. Further support was provided by grants from:—the ANR (ANR-12-SAMA-0004, AAPG2019—GeBra), the Eranet Neuron (AF12-NEUR0008-01—WM2NA; and ANR-18-NEUR00002-01—ADORe), the Fondation de France (00081242), the Fondation pour la Recherche Médicale (DPA20140629802), the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012, the Fondation de l’Avenir (grant AP-RM-17-013), the Fédération pour la Recherche sur le Cerveau; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1) and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence.
Author information
Authors and Affiliations
Consortia
Contributions
GG: formal analysis; investigation; writing—original draft. RMM: conceptualization; formal analysis; investigation; writing—original draft. AL: writing—review and editing. AS: writing—review and editing. FGB: writing—review and editing. PF: supervision; writing—review and editing. LS: supervision; writing—review and editing. TB: writing—review and editing. GJB: writing—review and editing. ALWB: writing—review and editing. SD: writing—review and editing. AG: writing—review and editing. HG: writing—review and editing. PG: writing—review and editing. AH: writing—review and editing. RB: writing—review and editing. JLM: data curation; investigation; resources; writing—review and editing. MLPM: writing—review and editing. EA: data curation; writing—review and editing. FN: data curation; investigation; resources; writing—review and editing. DPO: data curation; writing—review and editing. LP: data curation; investigation; resources; writing—review and editing. SH: writing—review and editing. NH: writing—review and editing. JHF; data curation; investigation; resources; writing—review and editing. MNS: data curation; investigation; resources; writing—review and editing. NV: writing—review and editing. HW: data curation; investigation; resources; writing—review and editing. RW: data curation; investigation; resources; writing—review and editing. GS: data curation; investigation; resources; project administration; writing—review and editing. HL: conceptualization; methodology; software; validation; data curation; writing—review and editing; visualization; supervision. HV: conceptualization; formal analysis; validation; writing—original draft; visualization; supervision.
Corresponding author
Ethics declarations
Conflict of interest
Tobias Banaschewski served in an advisory or consultancy role for eye level, Infectopharm, Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Roche, and Takeda. He received conference support or speaker’s fee by Janssen, Medice and Takeda. He received royalities from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press; the present work is unrelated to these relationships. Dr. Barker has received honoraria from General Electric Healthcare for teaching on scanner programming courses. Dr. Poustka served in an advisory or consultancy role for Roche and Viforpharm and received speaker’s fee by Shire. She received royalties from Hogrefe, Kohlhammer and Schattauer. The present work is unrelated to the above grants and relationships. The other authors report no biomedical financial interests or potential conflicts of interest.
Ethics approval
The study was performed in accordance with the Declaration of Helsinki. Local ethics committees approved all study procedures.
Consent to participate
Written informed assent and consent were obtained, respectively, from all adolescents and their parents after complete description of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the IMAGEN Consortium group are given in Acknowledgements.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gros, G., Miranda Marcos, R., Latrille, A. et al. Whole-brain gray matter maturation trajectories associated with autistic traits from adolescence to early adulthood. Brain Struct Funct 229, 15–29 (2024). https://doi.org/10.1007/s00429-023-02710-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-023-02710-2