Abstract
Satb1 and Satb2 belong to a family of homeodomain proteins with highly conserved functional and regulatory mechanisms and posttranslational modifications in evolution. However, although their distribution in the mouse brain has been analyzed, few data exist in other non-mammalian vertebrates. In the present study, we have analyzed in detail the sequence of SATB1 and SATB2 proteins and the immunolocalization of both, in combination with additional neuronal markers of highly conserved populations, in the brain of adult specimens of different bony fish models at key evolutionary points of vertebrate diversification, in particular including representative species of sarcopterygian and actinopterygian fishes. We observed a striking absence of both proteins in the pallial region of actinopterygians, only detected in lungfish, the only sarcopterygian fish. In the subpallium, including the amygdaloid complex, or comparable structures, we identified that the detected expressions of SATB1 and SATB2 have similar topologies in the studied models. In the caudal telencephalon, all models showed significant expression of SATB1 and SATB2 in the preoptic area, including the acroterminal domain of this region, where the cells were also dopaminergic. In the alar hypothalamus, all models showed SATB2 but not SATB1 in the subparaventricular area, whereas in the basal hypothalamus the cladistian species and the lungfish presented a SATB1 immunoreactive population in the tuberal hypothalamus, also labeled with SATB2 in the latter and colocalizing with the gen Orthopedia. In the diencephalon, all models, except the teleost fish, showed SATB1 in the prethalamus, thalamus and pretectum, whereas only lungfish showed also SATB2 in prethalamus and thalamus. At the midbrain level of actinopterygian fish, the optic tectum, the torus semicircularis and the tegmentum harbored populations of SATB1 cells, whereas lungfish housed SATB2 only in the torus and tegmentum. Similarly, the SATB1 expression in the rhombencephalic central gray and reticular formation was a common feature. The presence of SATB1 in the solitary tract nucleus is a peculiar feature only observed in non-teleost actinopterygian fishes. At these levels, none of the detected populations were catecholaminergic or serotonergic. In conclusion, the protein sequence analysis revealed a high degree of conservation of both proteins, especially in the functional domains, whereas the neuroanatomical pattern of SATB1 and SATB2 revealed significant differences between sarcopterygians and actinopterygians, and these divergences may be related to the different functional involvement of both in the acquisition of various neural phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The special AT-rich sequence-binding proteins 1 and 2 (SATB1 and SATB2) belong to a family of homeodomain proteins, with a SATB domain and two CUT domains, and a homeodomain for DNA binding (Dickinson et al. 1992; Cai et al. 2003; FitzPatrick et al. 2003; Britanova et al. 2005). In particular, the CUT domain binds to specific AT-rich matrix-binding regions (Dickinson et al. 1997), which attach to the nuclear matrix, separating chromatin into topologically independent loop domains. In particular, they bind to multiple sites within chromatin loop domains and modulate their organization and structure, modifying the transcription of various genes at different levels, including in the brain (Ciejek et al. 1983; Vaughn et al. 1990; Alvarez et al. 2000; Cai et al. 2003; Dobreva et al. 2003, 2006; Britanova et al. 2005; Ahn et al. 2010). Therefore, in contrast to classical transcription factors that bind to individual target genes, SATB family members regulates target genes in a context-dependent manner, depending on its genomic occupancy and potential to form a complex with multiple partners. Moreover, this probably occurs thanks to this functional flexibility, with a highly conserved pattern in evolution.
It has been described a 61% homology between SATB1 and SATB2 at amino acid level, and in the mouse, no other homologues have been identified (Britanova et al. 2005). Firstly, it was described SATB1, regulating the expression of multiple genes in the maturating T-cells, as a global regulator of thymocyte differentiation (Dickinson et al. 1992; Yasui et al. 2002; Cai et al. 2003), but it was also detected in the fetal brain. SATB2 was described in the SATB2-associated syndrome, an autosomal dominant neurodevelopmental disorder caused by alterations in the SATB2 gene, which is a multisystem disorder characterized by significant neurodevelopmental compromise with limited to absent speech, behavioral issues, and craniofacial anomalies (reviewed in Zarate et al. 2017, 2019). In addition, its regulatory role has been shown to be crucial during early vertebrate embryogenesis (Pradhan et al. 2021). In the brain, SATB2 has been implicated in the development of different regions like the mouse neocortex (Alcamo et al. 2008; Britanova et al. 2008; Leone et al. 2015; McKenna et al. 2015), different hypothalamic subpopulations (Kurrasch et al. 2007; Blackshaw et al. 2010; Bedont et al. 2015; Ferran et al. 2015; Glendining et al. 2020; Zhang et al. 2021), and in the function of the parabrachial area (Fu et al. 2019; Jarvie et al. 2021; Karthik et al. 2022). Its expression and functional domains are highly conserved in humans, mice, chick, and zebrafish (Ahn et al. 2010; Sheehan-Rooney et al. 2010). Moreover, in the case of the SATB1 and SATB2 expression in the brain, particularly well described in mice, their expression patterns apparently do not overlap (Britanova et al. 2005).
Thus, considering the functional importance and versatility of these proteins from early developmental stages and in a multitude of systems including the brain, extending its knowledge to basal fish models may provide important insights. It is known that comparative expression patterns of transcription factors involved in the acquisition of the identity of specific neuronal phenotypes, or of major brain subdivisions, constitute an extremely important source of information in the analysis of brain evolution, but also improve the understanding of how the brain is organized and functions. Thus, in this context of a conserved gene family, there are currently no data on the expression pattern of SATB1 in non-mammalian models, and data on SATB2 are very scarce. In particular, there is a lack of data on the expression and function of both proteins at the base of the evolutionary scale (except SATB2 in zebrafish). Furthermore, in the case of fishes, the high diversity found implies that many of the evolutionary lineages are poorly studied anatomically. Therefore, in the present study, we have selected representative species of actinopterygian and sarcopterygian fishes that allow us to identify differences in critical evolutionary points (see Fig. 1A). On the one hand, lungfishes constitute the most basal sarcopterygian model and the only non-tetrapod anamniote sarcopterygian, therefore the closest living relatives of all tetrapods. On the other hand, during the Devonian period, basal actinopterygian fishes were divided into the classes Cladistia (polypteriform fishes) and Actinopteri, which gave rise to the subclass Neopterygii (holostean and teleost fishes), and sturgeons (Broughton et al. 2013; Betancur-R et al. 2013; Hughes et al. 2018; Du et al. 2020). Therefore, in order to infer the characteristics specific to ancestral Osteichthyes, and to the transition between actinopterygians and sarcopterygians, as well as the evolutionary adaptations of each group, the first objective of the present study was to perform an in-depth analysis of the SATB protein sequences in these evolutionarily key species. The second, and main goal was to analyze in detail their neuroanatomical distribution in the brain of the mentioned models. Additionally, we phenotypically characterized these neurons by double immunolabeling techniques with the enzyme tyrosine hydroxylase (TH), as the main catecholaminergic marker (mainly dopaminergic and noradrenergic), serotonin (5-HT), and the transcription factor Orthopedia (Otp), highly expressed in particular regions of the amygdaloid complex and the hypothalamus (Puelles and Rubenstein 2003; Domínguez et al. 2013, 2014, 2015; Moreno et al. 2012).
A Cladogram showing the phylogenetic relationships of the four major groups of vertebrates: agnathans, chondrichthyans (cartilaginous fishes), actinopterygians (ray-finned fishes), and the sarcopterygian radiation of lobe-finned fishes. B Number of animals (n) and species used in the present study. The fishes were purchased from the authorized commercial supplier PezyCia (Madrid, Spain)
Materials and methods
Analysis of the sequence
We run a BLAST PROTEIN of the whole SATB1 and SATB2 protein sequences of Homo sapiens (H.s; taxid: 9606), Protopterus annectens (P.a; taxid: 7888), Lepisosteus oculatus (L.o; taxid: 7918), Danio rerio (D.r; taxid: 7955), Acipenser ruthenus (A.r; taxid: 7906) and Polypterus senegalus (P.s; taxid: 55291). CLUSTALW was used for multiple sequence alignments (Figs. 2 and 3). The epitope regions of the antibodies used in the present study (Table 1) were identified in the whole protein sequences of the models under analysis and BLAST PROTEIN and CLUSTALW analyses were performed for multiple sequence alignments (Table 2).
Full-length SATB1 protein sequence alignment in Homo sapiens (H.s) as a query, and Protopterus annectens (P.a), Lepisosteus oculatus (L.o), Danio rerio (D.r), Acipenser ruthenus (A.r) and Polypterus senegalus (P.s) using CLUSTALW. Identical amino acids are shown with an asterisk (*). The colon (:) and the dot (.) indicate a conserved substitution, and a semi-conserved substitution respectively. The SATB domain is shown in blue, the CUT domains are shown in purple and the homeodomain in yellow. The epitope sequence recognized by the Satb1/2 antibody (Abcam. Catalog reference ab51502) is underlined in red and the epitope sequence recognized by the Satb1 antibody (Santa Cruz Biotechnology. Catalog reference sc-376096) is underlined in green
Full-length SATB2 protein sequence alignment in Homo sapiens (H.s) as a query, and Protopterus annectens (P.a), Lepisosteus oculatus (L.o), Danio rerio (D.r), Acipenser ruthenus (A.r) and Polypterus senegalus (P.s) using CLUSTALW. Identical amino acids are shown with an asterisk (*). The colon (:) and the dot (.) indicate a conserved substitution, and a semi-conserved substitution respectively. The SATB domain is shown in blue, the CUT domain is shown in purple and the homeodomain in yellow. The epitope sequence recognized by the Satb1/2 antibody (Abcam. Catalog reference ab51502) is underlined in red and the epitope sequence recognized by the Satb1 antibody (Santa Cruz Biotechnology. Catalog reference sc-376096) is underlined in green
Tissue handling
Representative species of the main sarcopterygian and actinopterygian fish were used (see Fig. 1). The animals were obtained from licensed suppliers (PezyCia, Madrid, Spain). Information about the number of the specimens used in the present study is shown in Fig. 1B. The animals were deeply anesthetized with 0.1% tricaine methanesulfonate solution (MS222, pH 7.4; Sigma-Aldrich, Steinheim, Germany) by immersion.
The brains and rostral spinal cords were removed after fixation by transcardial perfusion of the animals with 4% paraformaldehyde in a 0.1 M phosphate buffer (PB, pH 7.4) and subsequently cryoprotected in a solution of 30% sucrose in PB for 4–6 h at 4 ºC. The brains and spinal cords were cut at 30–40 μm thickness, in transverse or sagittal planes, on a freezing microtome, after embedded in a solution of 20% gelatin with 30% sucrose in PB.
Immunofluorescence analysis
Single and combined immunofluorescence for detection of SATB1 or SATB1/2 and OTP, TH, or 5-HT was carried out. Information about the commercial specifications, immunogen information, the dilution used, and the secondary antibodies is detailed in Table 1. In all cases, the antibodies were diluted in PB containing 0.5% Triton X-100 and the sections were mounted with fluorescence mounting medium (Santa Cruz; SC-24941 or Vectashield H-1500). All antibodies were tested by omission or by incubation with preimmune mouse or rabbit sera instead of the primary antibody and no residual staining was observed.
Imaging
The sections were analyzed with the microscopes: Olympus BX51, Olympus FV 1200 and Leica sp-2 AOBS confocal. The figure preparation was done with Adobe Photoshop CS6 (Adobe Systems, San Jose, CA) and Canvas X (ACD Systems, Canada).
Results
Sequence analysis
The models analyzed by NCBI BLAST PROTEIN and CLUSTALW were Homo sapiens (H.s; taxid: 9606) as a query, and Protopterus annectens (P.a; taxid: 7888), Lepisosteus oculatus (L.o; taxid: 7918), Danio rerio (D.r; taxid: 7955), Acipenser ruthenus (A. r; 7906) and Polypterus senegalus (P.s; taxid: 55291). The analysis was carried out based on the whole sequence proteins that were aligned using CLUSTALW to establish comparisons of the SATB1 (Fig. 2) and SATB2 (Fig. 3) proteins among these species. In addition, the epitope regions of the antibodies used in the expression patterns description were identified in the whole protein sequences of the models under analysis (see color code in Figs. 2 and 3).
The SATB1 sequence alignment in BLAST indicated that the lungfish P. annectens shares 83,63% sequence identity with human SATB1, the holostean L. oculatus 81,10%, the teleostean D. rerio 72,48%, the sturgeon A. ruthenus 79,08%, and the polypteriform P. senegalus 81,16%. In all cases, the sequences analyzed showed a SATB domain, two CUT domains and a homeobox domain (see color code in Fig. 2). In particular, the conservation of these domains is especially high in all models.
The SATB2 sequence alignment in BLAST indicated that the lungfish P. annectens share 88,18% sequence identity with human SATB2, the holostean L. oculatus 64,97%, the teleostean D. rerio 68,25%, the sturgeon A. ruthenus 75,49%, and the polypteriform P. senegalus 75,16%. In all cases, the sequences analyzed also showed a SATB domain, two CUT domains and a homeobox domain (see color code in Fig. 3). Comparatively, the SATB2 sequence showed a lower degree of conservation than SATB1 with respect to the human sequence in all models analyzed.
The subsequent analysis to ensure the reliability of the expression analysis performed was to examine the epitopes of the SATB1 and SATB2 protein sequences, from which the antibodies used in the present study were generated (see Tables 1 and 2).
In the case of the antibody developed against SATB1/2 (monoclonal mouse anti-Satb1/2. Abcam. Catalog reference: ab51502), when we compared this epitope sequence with the complete SATB1 sequence (see Table 2), we observed that the alignment score is around 50 (including human, dropping to 47 in zebrafish), and the percent identity is around 60%. The alignment to the SATB2 sequence revealed that this antibody is recognizing SATB2 in all fish models (see Table 2), being especially striking the case of lungfish, where the score rises to 84 and the percent identity to 85%, which confirms the high degree of conservation of the sequence. Therefore, it cannot be excluded that this antibody can recognize both proteins, although in the case of SATB1 with much lower affinity, attending to the sequence.
The comparison of the sequence of the epitope developed specifically against SATB1 (monoclonal mouse anti-Satb1. Santa Cruz Biotechnology; Catalog reference: sc-376096) revealed that this antibody is recognizing SATB1 in all models (the score is around 80 in all cases, being the lowest in zebrafish with 67; see Table 2). The comparison of this epitope with SATB2 showed that the alignment is around 20 in all cases (including humans, which drops to 27 and the percent identity to 36.8%), and the percent of identity in actinopterygians results in no significant similarity. This strongly supports the specificity of this antibody in the exclusive identification of SATB1 protein.
Although the analyses have demonstrated that both proteins SATB1 and SATB2 are present in the teleost D. rerio, the antibody against the former protein yielded no results in the immunofluorescence experiments. Considering that the gene Satb1 is present in D. rerio, annotated in the forms a and b (ZFIN:ZDB-GENE-031010-36 and ZFIN:ZDB-GENE-030131-7105), and that the antibody against SATB1 used showed a high degree of similarity in the epitope sequence (see Table 2 and Fig. 2), we believe this discrepancy could be due to an effect of protein folding in this teleost model, as opposed to the rest of the models analyzed. Therefore, these data have not been included in Table 3.
Expression pattern
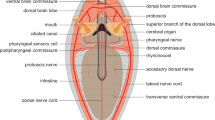
The neuroanatomical nomenclature used in the present work is similar to previous studies with the same sarcopterygian and actinopterygian fish species (López et al. 2019, 2022; Lozano et al. 2019; Jiménez et al. 2020). The distribution of the immunoreactive labeling for SATB1, SATB1/2, OTP, TH and 5-HT (Figs. 4, 5, 6, 7, 8, 9) was analyzed in relation to the main prosomeric brain regions (Puelles and Rubenstein 2015), adapted for each model. In addition, the SATB1 and SATB2 expression patterns are represented in sagittal drawings, with special reference to the neuromeric (segmental) regionalization of the brain (Fig. 10) and summarized in Table 3.
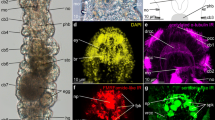
Photomicrographs of transverse (a–d, f–m) or sagittal (e) double-labeled sections through telencephalic areas of Protopterus dolloi (P.d), Danio rerio (D.r), Lepisosteus oculatus (L.o), Polypterus senegalus (P.s), and Acipenser ruthenus (A.r), showing the location of SATB1/2 (green color) alone or in combination with Otp or TH (red color) immunoreactivity. The markers and the species are indicated in each image. In the sagittal section the rostrocaudal and dorsoventral axis are indicated by arrows. a SATB1-ir cells in the glomerular layer of the olfactory bulb of A. ruthenus. b, c SATB1/2-ir cells in the pallium and subpallium of P. dolloi. d–f Subpallial derivatives of actinopterygian fishes labeled for SATB1/2. g–i The medial amygdala in P. dolloi, labeled for SATB1/2. j–m Caudal subpallial areas and the preoptic area, showing SATB1/2 immunoreactivity; empty arrowheads in (k) point to double labeled cells, white arrowheads in (l) and (m) point to TH-ir cells in POA and Otp-ir cells in Vp respectively. For abbreviations, see list. Scale bar: a, b, e, f = 200 µm; c, d, g = 100 µm
Photomicrographs of transverse (a–e, f–i, k) and sagittal (f, j) double-labeled sections of the preoptic area of Protopterus dolloi (P.d), Erpetoichthys calabaricus (E.c), Danio rerio (D.r), Lepisosteus oculatus (L.o), and Polypterus senegalus (P.s) showing the location of SATB1/2 or SATB1 (green color) alone or in combination with Otp or TH (red color) immunoreactivity. The markers and the species are indicated in each image. In the sagittal sections the rostrocaudal and dorsoventral axis are indicated by arrows. a, b SATB1/2 labeling in the POA of lungfish. c SATB1/2-ir cells in the rostral portion of POA of A. ruthenus; white arrowhead points to SATB1/2-ir cells in POAr. d–f SATB1/2 immunoreactivity in the POA of cladistians; empty arrowheads in (d) and (e) point to cells also labeled for TH. g SATB1-ir cells in the POA of P. senegalus; empty arrowhead points to double labeled cells. h, i SATB1/2-ir cells in the POA of L. oculatus; empty arrowhead in (h) and (i) points to double labeled cells. j, k SATB1/2-labeled cells in the POA of D. rerio; the line in (j) marks the level of (k), empty arrowheads point to double labeled cells. For abbreviations, see list. Scale bars: = 200 µm
Photomicrographs of transverse double-labeled sections through hypothalamic and diencephalic areas of Protopterus dolloi (P.d), Polypterus senegalus (P.s), Acipenser ruthenus (A.r), Danio rerio (D.r), and Lepisosteus oculatus (L.o) showing the location of SATB1/2 or SATB1 (green color) alone or in combination with Otp or TH (red color) immunoreactivity. The markers and the species are indicated in each image. a–c SATB1/2 labeling in cells of the subparaventricular hypothalamic region (a, b) and the prethalamic and thalamic areas (c) of P. dolloi. d SATB1/2 cells in the rostral part of the subparaventricular region of P. senegalus. e, f Immunoreactive cell nuclei in the preoptic area and the subparaventricular region of A. ruthenus. g SATB1/2-ir cells in the subparaventricular region of D. rerio; empty arrowheads point to double labeled cells. h Labeled cells in the prethalamus within the alar prosomere 3 of the lungfish. i Tuberal hypothalamic region of P. senegalus showing SATB1-ir cells. j SATB1 labeling in the prethalamus and posterior tubercle of the sturgeon. k, l SATB1 immunoreactivity in cells of the thalamus and pretectum of L. oculatus (k) and P. senegalus (l). m SATB1-ir cells in the mamillary region of P. senegalus; empty arrowhead points to double labeled cells. n–q Labeled cells in the posterior tubercle (n) and the tuberal hypothalamic region (o–q) of the lungfish; empty arrowheads in q point to double labeled cells. For abbreviations, see list. Scale bar: a–k, n–t = 200 µm; m, l = 100 µm; u = 50 µm
Photomicrographs of transverse single-labeled sections through mesencephalic areas of Polypterus senegalus (P.s), Lepisosteus oculatus (L.o), Acipenser ruthenus (A.r), and Danio rerio (D.r) showing the location of SATB1/2 or SATB1 immunoreactivity. The markers and the species are indicated in each image. a–f SATB1-ir cells in the periventricular layer of the optic tectum (spv), torus semicircularis and tegmentum of P. senegalus (a), L. oculatus (b), and A. ruthenus (c, d), the latter also with labeled cells in more superficial layers of the optic tectum; white arrowheads point to cells in spv, empty arrowheads in (c) and (d) point to cells in other tectal layers. e, f SATB1/2-ir cells in the optic tectum and tegmentum of D. rerio; arrowheads point to periventricular cells. For abbreviations, see list. Scale bars = 200 µm (c), 100 µm (a, b, d, e), 50 µm (f)
Photomicrographs of transverse single- and double-labeled sections through mesencephalic and rhombencephalic areas of Protopterus dolloi (P.d), Polypterus senegalus (P.s), and Danio rerio (D.r) showing the location of SATB1/2 or SATB1 (green color) alone or in combination with TH, Otp or 5-HT (red color) immunoreactivity. The markers and the species are indicated in each image. a, b SATB1/2-ir cells in the torus semicircularis and mesencephalic tegmentum (a), and in the central gray (b) of P. dolloi. c Populations of SATB1-ir cells in the central gray and superior reticular nucleus of P. senegalus. d SATB1/2-labeled cells in the central gray of D. rerio. For abbreviations, see list. Scale bars = 200 µm (c), 100 µm (a, b, d, e), 50 µm (f)
Photomicrographs of transverse single- and double-labeled sections through rhombencephalic areas of Protopterus dolloi (P.d), Acipenser ruthenus (A.r), Polypterus senegalus (P.s), and Lepisosteus oculatus (L.o) showing the location of SATB1/2 or SATB1 (green color) alone or in combination with TH, 5-HT or Otp (red color) immunoreactivity. The markers and the species are indicated in each image. a–c SATB1/2-ir cells in the reticular formation of P. dolloi. d Population of SATB1-ir cells in the facial lobe and solitary tract nucleus of A. ruthenus. e SATB1/2-ir cells in the interpeduncular nucleus of P. senegalus. f, g Populations of SATB1/2-ir cells in the ventral octavolateral area (f) and the median reticular nucleus (g) of L. oculatus. h, i Reticular cells labeled with SATB1 in P. senegalus (h) and A. ruthenus (i); arrowheads in (h) point to double labeled cells. For abbreviations, see list. Scale bars = 200 µm (c), 100 µm (a, b, d, e), 50 µm (f)
Schematic representation of SATB1 (A) and SATB2 (B) expression patterns in a model vertebrate. The schemes represent the main regions of expression found in the present study. The particular exceptions have not been included for simplicity but are detailed in the text and in Table 3. The correspondence of the color code is indicated on the right. The anatomical representation does not correspond to any particular model. See list for abbreviations
Distribution of SATB1 and SATB2
Telencephalon: SATB1/2 expression was analyzed in the telencephalon in combination with markers such as TH and OTP (Figs. 4 and 5). The olfactory bulbs showed only SATB1-immunoreactive (SATB1-ir) cell nuclei in the glomerular layer of cladistians species, A. ruthenus and L. oculatus, in a lateral position (Fig. 4a).
In the telencephalon of the lungfish Protopterus dolloi, labeling of SATB1 and SATB2 was observed in the ventral and lateral regions of the pallium (in Fig. 4b, c), while the actinopterygian species studied lacked any pallial labeling. In the subpallium, the lateral septal area and the bed nucleus of stria terminalis in lungfish (Fig. 4c) and its homolog in ray-finned fishes—the ventral part of the ventral telencephalic area (Vv)—showed cell nuclei labeled with both antibodies in all fish species studied, with the exception of cladistians for SATB1 (Fig. 4d–f). In addition to this, codistributing with TH-ir cells, the caudal levels of the dorsal part of the ventral telencephalic area (Vd) housed scattered cells labeled with both antibodies in all actinopterygian fishes studied, with the exception of L. oculatus, whose Vd showed no SATB2 cells (Fig. 4d–f). In contrast, its homolog located in the striatum of lungfish was devoid of any labeling. At caudal telencephalic levels, scattered SATB1- and SATB2-ir cells were detected in the region described as medial amygdala in lungfish (Fig. 4g–i), and in its homolog in actinopterygian fishes, the supracommissural and posterior nuclei of the ventral telencephalic area (Vs-Vp) (Fig. 4j, k, m), a region that was clearly identified in combination with TH (Fig. 4i–k) and OTP (Fig. 4h, m). In particular, certain degree of colocalization of SATB2 and TH was detected in this region in cladistians (see empty arrowheads in Fig. 4k).
The preoptic area of all species of bony fish studied showed a prominent SATB1/2 labeling (Figs. 4j–m, 5 and 6e). Two subpopulations can be observed in this region: a caudal one (following the prosomeric view; dorsal in classical view), more numerous and densely packed, and without colocalization with the TH-ir cells of this region, and a rostral one (POAr; ventral in classical view), in the acroterminal domain, many of whose cells were doubly labeled with TH (Fig. 5d, e, h–k). Moreover, the combination with TH and OTP revealed the boundaries with the paraventricular domain of the alar hypothalamus (Fig. 5a–c, f, g).
Hypothalamus and diencephalon: In the alar hypothalamus, chondrostean (Fig. 5c) and cladistian species presented some scattered SATB1-ir cells in the dorsal paraventricular region, and in this former, some of them were doubly labeled with OTP (see empty arrowhead in Fig. 5g). In the subparaventricular region, by contrast, SATB1/2-ir cells were observed in the rostral part of the suprachiasmatic nucleus of all fish species studied (Fig. 6a, b, d, f), some of which were doubly labeled with the TH antibody in D. rerio (Fig. 6g). Regarding the basal hypothalamus, the cladistian species housed a population of SATB1-labeled cells in the tuberal region (Fig. 6i), while in the case of the lungfish this hypothalamic region showed SATB1/2-ir cells (Fig. 6o), some of them colocalizing with OTP in the rostral portion, but only at caudal levels (Fig. 6p, q). In addition, SATB1-ir cells were observed in the mamillary region of the chondrostean A. ruthenus and the cladistian species exclusively, the latter possessing certain degree of colocalization with OTP (Fig. 6m).
In the diencephalon, SATB1-ir labeling was detected in cell nuclei of the alar prethalamic, thalamic, and pretectal areas of all fish species studied (excluding D. rerio) (Fig. 6j–l), being the lungfish the only species that also showed SATB2 labeling in the prethalamus and the thalamus (Fig. 6c, h). In addition to these alar diencephalic domains, the basal posterior tubercle of the chondrostean A. ruthenus presented a periventricular population of SATB1-ir cells (Fig. 6j), while in the case of the lungfish the immunoreactive cell nuclei of this region were labeled with SATB1/2 (Fig. 6n).
Brainstem: Within the alar midbrain, many SATB1-ir cells were observed in the tectal periventricular layer of cladistian species, the holostean L. oculatus and the chondrostean A. ruthenus, stretching in the latter into more superficial layers of the optic tectum, but leaving the stratum opticum devoid of labeled cells (Fig. 7a–d). In addition, the optic tectum of the teleost D. rerio housed scattered SATB1/2-ir cells in the periventricular layer (Fig. 7e, f). Apart from the optic tectum, the torus semicircularis and the mesencephalic tegmentum also presented populations of cells labeled for SATB1 in the non-teleost actinopterygian species (Fig. 7a–c), while these areas showed SATB1/2 immunoreactivity in the lungfish (Fig. 8a). In the case of D. rerio, only the mesencephalic tegmentum was labeled with SATB1/2 (Fig. 7e).
In the rostral rhombencephalon, SATB1 labeling was detected in cells of the central gray of all fishes studied (Fig. 8c), with the exceptions of D. rerio and P. dolloi, whose central gray presented cells labeled for SATB1/2 (Fig. 8b, d). Strikingly, SATB1/2-ir cells were observed in the interpeduncular nucleus of the cladistians species, ventrally to the superior raphe nucleus (Fig. 9e), while the holosteans L. oculatus showed labeled cells in the ventral octavolateral area (Fig. 9f). The reticular formation showed in its superior and median portions SATB1 immunoreactivity in cladistians and A. ruthenus (Fig. 9h, i), and SATB1/2 in L. oculatus and the lungfish (Figs. 8c and 9a, c, g). Only in the case of the lungfish the inferior portion also presented labeled cells (Fig. 9c), while cladistians were the only species to colocalize SATB1 and OTP in cells of the reticular formation, specifically in its median portion (Fig. 9h). In mid to caudal alar rhombencephalic levels, a remarkable population exclusively of SATB1-ir cells was observed in the facial lobe and solitary tract nucleus of non-teleost actinopterygian fishes, distinct of the TH-ir cells housed in this nucleus (Fig. 9d). Finally, no immunolabeling was observed in the spinal cord of any species studied.
Discussion
Protein sequence analysis and technical considerations
Comparing the SATB1 sequences in all fish models analyzed we found that, in general terms, it is very conserved in all of them, and in particular the functional domains (see Fig. 2). For the SATB2 sequence, it showed a lower degree of similitude compared to SATB1, especially in the case of teleosts and holosteans (see Fig. 3). However, the degree of similarity and therefore the conservation of the sequence is high.
A previous study about the SATB2 role in the craniofacial development provided a detailed insight into the conserved protein sequence and the expression pattern in mouse, chick and zebrafish (Sheehan-Rooney et al. 2010). The characterization of the full-length SATB2 transcript in mouse, chick and zebrafish compared to human, indicated, as in our present results, that the predicted protein sequence of SATB2 is conserved, in particular, the SATB, CUT and homeobox domains (Sheehan-Rooney et al. 2010), suggesting that the functional implication is also conserved. The expression analysis showed that the spatial and temporal pattern of SATB2 in the species analyzed correlates with those structures affected in human SATB2 mutated patients (Britanova et al. 2006; Dobreva et al. 2006; see discussion in Sheehan-Rooney et al. 2010). In addition, after Satb2 morpholino injections in zebrafish, migration defects during gastrulation have been described at early developmental stages (Ahn et al. 2010), although later developmental alterations were not described, most likely due to unviability of the zebrafish embryos.
In the analysis of epitope sequences performed in the present study, the SATB1 antibody was designed against an amino acids sequence from the center of the SATB1 protein of human (see Fig. 2). When we analyzed the sequence in all models, including human, we observed that this antibody recognizes SATB1 with high specificity in the models analyzed, but not SATB2. Therefore, we conclude that the specificity we observed in the results is very reliable. In the case of zebrafish, we believe that the fail in the immunofluorescence experiments is due to an effect of protein folding in this teleost model as opposed to the rest of the fishes analyzed, since based on the sequence analysis we know that SATB1 is present (the gen is annotated in the forms a and b: ZFIN:ZDB-GENE-031010-36 and ZFIN:ZDB-GENE-030131-7105 respectively) and it shows a high degree of similarity in the epitope recognized by the antibody (present results).
The SATB1/2 antibody under analysis was designed against a recombinant fragment corresponding to human SATB2 in the C-terminal end of the protein (see Table1 and Fig. 3). In particular, this antibody recognizes amino acids starting at 601 in the C-terminus of the human SATB2 protein (Uniprot: Q9UPW6). According to our present results, this region of the protein is very similar, in the species tested including human, to that present in the SATB1 protein (see Table 2). In addition, it was recently indicated by the commercial supplier in the specifications of the product that this antibody targets SATB1 and SATB2 proteins. According to our results, it is possible that this antibody recognizes both proteins in all species studied, although SATB2 with a higher degree of specificity.
The present protein analyses confirmed the high degree of conservation of both SATB1 and SATB2 proteins, in particular their functional domains (see color codes in Figs. 2 and 3), and therefore, the reliability of the results obtained by immunohistochemistry is very high, but we cannot exclude that we are detecting both populations with the SATB1/2 antibody.
The neuroanatomical expression pattern
In the present comparative study, we have analyzed the immunolabeling pattern of SATB1 and SATB2 in the brain of key fishes, using in all cases the same commercial antibodies under the same technical conditions (see “Materials and methods” section).
SATB1 and SATB2 expression patterns in the brain have been described in detail in mouse (Huang et al. 2011, 2013), but in these studies, based on the antibodies used for their analyses, we consider that the description of SATB2 may correspond to both proteins. This is because many of the currently commercially available antibodies against SABT2 are designed (like the abcam antibody used in the present study) against a region of the protein very similar to that of SATB1. We will discuss each case in detail. In other amniotes, SATB2 expression has been correctly described in the pallium of chicken (García-Moreno et al. 2018), turtle (Suzuki and Hirata 2014), and alligator (Briscoe and Ragsdale 2018), but detailed data are lacking in the rest of the brain, as well as in anamniotes, except for zebrafish (Ahn et al. 2010; Pradhan et al. 2021). Moreover, it is particularly striking that there are no data on SATB1 expression in non-mammalian vertebrates. Thus, in the present study we conducted a comprehensive analysis of SATB1 and SATB2 immunolocalization in the brain of key osteichthyanfishes, selected because of their special evolutionary relationships (see Fig. 1A). In particular, we have generated a genoarchitectonic map of evolutionary SATB1 and SATB2 expressions that is consistent with the prosomeric model, but with notable differences in expression patterns between sarcopterygians and actinopterygians fishes that point to important divergent features in these vertebrate brain areas (Fig. 10). In the following sections, we will discuss our results in the context of existing data in amniotes and zebrafish for each main region of the brain.
Forebrain
Olfactory bulb and pallium. In the olfactory bulbs of mice it has been described Satb1 gene expression in both neuronal and non-neuronal cell populations (Chang and Parrilla 2016). However, at protein expression terms, in adult mouse no SATB1 positive neurons were described in the main and accessory olfactory bulbs (Huang et al. 2011). In our analysis we have detected the presence of a population of SATB1 cells in the olfactory bulb of actinopterygian fish, contrary to what has been observed in sarcopterygians, both in lungfish (present results) and in other tetrapod models (such as amphibians or reptiles; data not shown). This data is very interesting as it results in a discrepancy at this evolutionary point, but it also raises the need to analyze in other models of sarcopterygians what is happening during development, since it could be similar to mammals, in which its expression is lost in the adult stage, or the contrary, with this ancestral characteristic of actinopterygian fish lost in these non-mammalian models.
In the case of Satb1 in the adult mouse cortex, positive neurons were abundant in the deep layers of the neocortex, subiculum, and anterior olfactory nucleus (Huang et al. 2011). Satb2 has been shown to be expressed with other cell identity transcription factors (CITF genes) in birds, reptiles, and mammals (Nomura et al. 2018; Tosches et al. 2018; Tosches and Laurent 2019). In mammals, Satb2 was abundantly expressed in all six layers of the neocortex (Britanova et al. 2005, 2008; Alcamo et al. 2008; Nielsen et al. 2010; Balamotis et al. 2012; Zhang et al. 2012; Huang et al. 2013; Martins et al. 2021), especially in the intracortical projection cells through the corpus callosum (Paolino et al. 2018), repressing the extracortical projection neuron identity in these cells.
The present results in the sarcopterygian and actinopterygian models analyzed allow us to conclude that the extensive expression of SATB1 and SATB2 in the pallium is an acquired characteristic of sarcopterygians, since only in the case of lungfish we found a discrete expression in the pallial region, which is totally absent in the case of actinopterygians. The absence of SATB1 and SATB2 in the pallium of the actinopterygian fishes suggests a null participation of these transcription factors in the mechanisms implicated in the pallial formation, although additional data in development are necessary to confirm this idea. In evolutionary terms, it makes sense, since Satb2 has been implicated in the evolution of the neocortex through different mechanisms, but in particular it has been shown that during cortical neurogenesis the control of Satb2 expression timing is crucial. For example, in higher primates, the expansion of supragranular cell layers has been linked to the delayed appearance of the Satb2 protein (Otani et al. 2016; Paolino et al. 2020; Martins et al. 2021). Satb2 may also relate to additional evolutionary acquisitions, as it is the case for callosal projecting cells (Paolino et al. 2018). The earliest indication of a distinct pallial commissure in vertebrates comes from the African spotted lungfish, a basal member of the lobe-finned fish lineage (Northcutt and Westhoff 2011; Suárez et al. 2014). This commissural tract has been considered homologous among tetrapods (called the hippocampal commissure in mammals), whereas the corpus callosum is unique to placental mammals (Suárez et al. 2014, 2018). In this context, lungfish is the first model in evolution with pallial expression of SATB1- and SATB2-ir cells and a pallial commissure, in contrast to actinopterygians (present results). This is a very interesting difference, since its probable functional implication in the pallium evidences the key involvement of this gene in cortical evolution and opens an important line in the evolutionary analysis of the pallium.
Subpallium. In all models analyzed in the present study, scattered SATB1- and SATB2-ir cells have been observed in several subpallial structures (see Table 3 and Fig. 10). In the mouse subpallium, SATB1 has been reported in many positive neurons in the medial septal nucleus, the nucleus of the diagonal band, the bed nucleus of the stria terminalis (BST) and the substantia innominata (Huang et al. 2011). Moreover, SATB2 has been described in scattered cells in the BST and the diagonal band (Huang et al. 2013). As indicated above, it is possible that in these studies SATB2 expression also shows SATB1 expression due to the non-specificity of the antibody used. In the lungfish, we observed SATB1- and SATB2-ir cells in the BST and in the septum. In the case of actinopterygians, SATB1 and SATB2 expressing cells were detected in Vv, a region that may be genoarchitectonically and neurochemically interpreted as homologous of the septum and the BST (Moreno et al. 2012; González et al. 2014; López et al. 2016). Therefore, it seems to be a primitive and conserved feature.
Additionally, during development in mouse, it has been described that Satb1 is a key regulator of cortical interneuron development, required for the maturation of medial ganglionic eminencie (MGE)-derived cortical interneurons. In particular, Satb1 functions downstream of Lhx6 to control the transition of immature migrating interneurons into the differentiated somatostatin subtype (Denaxa et al. 2012). Moreover, conditional removal of Satb1 in mouse interneurons results in the loss of a majority of somatostatin-expressing cells across all cortical layers, as well as some parvalbumin-expressing cells in layers IV and VI, by postnatal day 21 (Close et al. 2012). We do not have data on SATB1 expression during development in the models analyzed, thus we do not know the expression in the MGE, but in adult, we did not find expression in pallial interneurons, in contrast to those observed in non-mammalian tetrapods (own unpublished data). However, the presence of somatostatin and parvalbumin-positive interneurons have been described in the pallium of Polypterus senegalus (Jiménez et al. 2020), therefore, although we have not detected SATB1 and SATB2 expression in the pallium of these adult models, we cannot rule out their involvement during development in the specification of these populations.
In the case of the amygdala, in mouse SATB1-positive neurons were described in the anterior part of the basolateral amygdaloid nucleus and, to a lesser extent, in the medial amygdaloid nucleus (Huang et al. 2011). Few SATB1- and SATB2-ir cells were observed in the caudal part of Vd of cladistians, chondrosteans and teleosts, a region that might correspond to the central amygdala of lungfishes and amphibians, based on the presence of nitrergic cells, among other features (Moreno and González 2005; González and Northcutt 2009; López et al. 2016). Still regarding the amygdaloid complex, scattered SATB1- and SATB2-ir cells have been detected in the medial amygdala of lungfish, as well as in the actinopterygian Vs and Vp regions (present results), suggesting that it seems highly conserved. The region identified in the caudal telencephalon of ray-finned fishes as Vs and Vp could be the actinopterygian representative of the medial amygdala of tetrapods, attending to the OTP expression and other neurochemical features (Moreno and González 2003; González et al. 2010; 2014; López et al. 2016, 2022), as it probably corresponds to the posterior and dorsal portions of the medial amygdala of zebrafish (Porter and Mueller 2020).
Preoptic area. The high presence of SATB1- and SATB 2-ir cells found in the preoptic region is probably the most conserved feature in all vertebrates analyzed in the present study. The regionalization of this territory is evident by the combination with TH, expressed in an extensive population in this region, although generally no double-labeled cells were found in any model. In addition, combined analysis with OTP, one of the main markers of the paraventricular hypothalamic region, revealed the boundary between both regions. Furthermore, SATB1 and SATB 2 expression was found in the acroterminal region of the preoptic area, including interestingly a significant population of double-labeled TH cells. This population is attributed to the acroterminal domain (Puelles and Rubenstein 2015), and it is important to note that this area is a direct derivative of (or occupies territory previously occupied by) the alar acroterminal organizer. Similarly, in mammals Satb2 has been described in basal regions in derivatives of the acroterminal domain, also derived from the acroterminal basal organizer (see below; Ferran et al. 2015), which suggests the likely important involvement in the specification of this domain. However, no SATB1 expression has been described in mouse (Huang et al. 2011).
Hypothalamus. In adult mice, SATB1 labeled neurons were observed in the medial tuberal nucleus, posterior hypothalamic area, ventral part of the premamillary nucleus and supramamillary nucleus (Huang et al. 2011), whereas SATB2 expression has been reported in the lateral hypothalamic area, the arcuate nucleus, the paraventricular nucleus (Huang et al. 2013) and the ventromedial nucleus (Glendining et al. 2020). Furthermore, in a recent re-evaluation of the hypothalamic organization it has been described that Satb2 expression during mouse development is restricted to the basal portion of the terminal hypothalamic prosomere, especially in the anterobasal nucleus, the tuberal domain and the ventromedial nucleus (Ferran et al. 2015). In our analysis, in the alar hypothalamus we detected cells very discretely in the paraventricular region of all models, probably migrated from the adjacent preoptic area. All models analyzed expressed SATB2-ir cells in the subparaventricular area, in particular in the suprachiasmatic nucleus (SC), but no double-labeled cells were observed within the TH population, with the exception observed in the lateral part of the SC of zebrafish. Thus, it seems to be a primitive and conserved feature, lost in mammals, and suggests the importance of this family of homeodomain proteins in the specification of this structure, or in any of its populations.
In the mouse basal hypothalamus, it has been described that, along with its involvement in the regionalization of the hypothalamus (Britanova et al. 2005; Kurrasch et al. 2007), Satb2 is important in the establishment of hypothalamic circuitry at specific times in development, in particular in the establishment of normal projections from the arcuate nucleus (Bouret and Simerly 2006). Furthermore, SATB2 is specifically expressed in the A12 group of hypothalamic dopaminergic neurons (Huang et al. 2013) and in the ventromedial hypothalamus (Kurrasch et al. 2007). In Satb2 KO mice, only the dopaminergic neurons in the arcuate nucleus were affected, due to a defective postmitotic differentiation in the absence of Satb2 (Zhang et al. 2021), revealing the involvement of Satb2 in the development of this population. Additionally, the arcuate nucleus characteristically expresses the transcription factor OTP (Acampora et al. 1999; Bardet et al. 2007), which is involved in somatostatin phenotype specification (Kurrasch et al. 2007; Lee et al. 2018). What we observed in the tuberal hypothalamic region, equivalent to the arcuate nucleus, is that SATB1- and SATB2-ir cells coexpress OTP, but not TH in lungfishes (present results), although it does in mammals (Zhang et al. 2021). Therefore, this region shows a peculiar expression that is likely related to the functional implication of these proteins in the specification of particular populations in this area. In our analysis we have detected the presence of a population of SATB1 cells in the mamillary region of the most basal models of actinopterygian fish, comparable to that described in mammals in the premamillary and supramamillary nucleus (Huang et al. 2011). Once again, in this case we are faced with the possibility of an ancestral feature missing in the rest of the scale, or a feature related to the adaptability of cell types. In this line, the hypothalamus of actinopterygian fishes shows many conserved and divergent structures and characteristics among vertebrates, like the inferior hypothalamic lobe and the saccus vasculosus, a peculiar sensor of seasonal changes in day length. Therefore, in this case, the evolutive acquisition, given the lack of expression of SATB1- and SATB2-ir cells in the basal hypothalamus of actinopterygian fishes, supports its importance in the specification of this region, and/or its connectivity in sarcopterygians. Moreover, this situation of both conserved and acquired characteristics makes this region an attractive subject for evolutionary studies.
Diencephalon. Within the diencephalon, only two thalamic nuclei have been described containing SATB1-positive neurons in mice, the centrolateral thalamic nucleus and the parafascicular thalamic nucleus (Huang et al. 2011). Among the models analyzed, only lungfish showed SATB1- and SATB2-ir cells in the thalamus, specifically, in scattered cells in the anterior and central nucleus. It seems to be an acquired feature by sarcopterygians, since the analyzed actinopterygian models lack SATB2-ir cells in this prosomere, but not SATB1 (see Table 3). This may be very interesting to analyze in terms of the implication of Satb2 in this functional circuit, since sarcopterygians are the only ones with pallial SATB1- and SATB2-ir cells, as opposed to actinopterygians, thus constituting an evolutionary novelty. The hodological relationships between the thalamus and the pallium, minor in the case of anamniotes, but more extensive in amniotes, could be related to this differential expression. Similarly, a novel evolutive acquisition observed exclusively in the brain of sarcopterygians, and so absent in all actinopterygians studied, is the presence of SATB2-ir cells in the prethalamus, in contrast to the actinopterygians, which only showed SATB1 (present results). From the point of view of cellular evolution and functional implications of these genes, this is very interesting, since it may be the case that SATB1 in these models is supplying the functions of both genes or that it is a necessity/acquisition at this moment of evolutionary transition.
Brainstem
In the mouse brainstem, the vast majority of the SATB1 cells were dopaminergic. In particular, SATB1-positive neurons were observed in the superior colliculus, the tegmental nuclei, the parabrachial nucleus, the substantia nigra pars compacta, the ventral tegmental area, the retrorubral field, the nucleus of the trapezoid body, the periolivary region, and the nucleus of the lateral lemniscus. In addition, the presence of SATB2 has also been reported in the ventral tegmental area, the laterodorsal tegmental nucleus, the dorsal raphe nucleus, the rostral periolivary region, and the parabrachial nucleus. It is also specifically expressed in serotonergic neurons in the dorsal part of the dorsal raphe nucleus (Huang et al. 2013). Therefore, there are some areas of coexpression of both proteins, although the SATB2 antibody could be detecting only SATB1 due to its specificity, as detailed in previous regions.
Within the midbrain of the analyzed models, only in sarcopterygians we have found SATB1- and SATB2-ir cells in the torus semicircularis and mesencephalic tegmentum, but SATB1 is expressed in both regions of all models. In the rhombencephalon, only the cladistian fishes show SATB2-ir cells in the raphe area, but no coexpression with serotonin was observed (present results). Although the dorsal raphe neurons have been described to express SATB2 in mouse (Huang et al. 2013), recent analyses by single-cell RNA sequencing and systematic in situ hybridization of the dorsal and medial raphe nuclei mapped specific clusters (differentially expressing neuropeptides, receptors, ion channels, and transcription factors), which did not include Satb2 (Ren et al. 2019). What has been described in postnatal mice, however, is that in the absence of 5-HT transporters, serotonergic innervation increased in the postnatal prefrontal cortex, but the Satb2-positive intracortical projection neurons decreased (Witteveen et al. 2013). Therefore, the possible presence of Satb2 in serotonergic cells of the raphe nuclei seems to be a novel and specific feature acquired in mammals.
Finally, in mammals, the parabrachial nucleus (Pb) is a mixed population, composed of different subpopulations, including Satb2-expressing neurons (Karthik et al. 2022). These subpopulations are hodologically related with many brain regions like the amygdala, the hypothalamus, and the hindbrain, participating in many different homeostatic functions. In addition, the Satb2 glutamatergic neurons of the Pb project to the cortical territories (Grady et al. 2020), in particular to regions associated with taste, including the gustatory thalamus and insular cortex (Jarvie et al. 2021). It is also interesting to note that projections from the insular cortex and the claustrum to subregions of the Pb containing cortical projection neurons have been described in mammals (Grady et al. 2020). Specifically, it has been observed that Satb2 neurons in the Pb respond to all five basic taste modalities (Jarvie et al. 2021), playing a critical role in sweet taste transduction (Fu et al. 2019). The sense of taste plays a key role in animal survival through an animal's reflex decision to swallow or reject a potential food item. In fish, taste information is mainly located in the secondary gustatory nucleus, originating in the isthmic region (Finger 2009). The absence of SATB1 and SATB2 cells in this nucleus in fish suggests that this acquisition appears later in evolution. However, given its functional importance and the degree of conservation in tetrapods (its existence has been demonstrated in amphibians and reptiles; data not shown), it is possible that some of the described populations lodged along the anterior reticular chain proximal to this region are involved in this functionality. Further experiments in this direction would be interesting.
Concluding remarks
We have analyzed the SATB1 and SATB2 protein sequences and their brain expression patterns in specific bony fish models at the base of gnathostomes. The first evidence shown is the high degree of conservation of these proteins in the base of evolution, in general in almost all the sequence, but especially in the functional domains, indicating that most probably the functional implications of these proteins are of great importance for survival, as demonstrated by their conservation in evolution. In this sense, these functional implications must be especially related to early developmental processes, as it has been described in mammals, since, as we can see in the analysis of the expression of both proteins in the brain, significant evolutionary divergences are observed between actinopterygians and sarcopterygians, even with the high degree of conservation they present. A striking example of this is the pallial region, where most probably both SATB1 and SATB2 are involved in the evolutionary process of this region, and more specifically in cortical expansion and cell type specification, due to the lack of expression in actinopterygian models, all of them with a pallium developed by a process of evagination.
Finally, in this sense, in the case of SATB2, alterations in this gene are related with the SATB2-associated syndrome, which causes craniofacial anomalies, among other pathologies (reviewed in Zarate et al. 2017). Similarly, the involvement of Satb2 in craniofacial development has been described in other models (Sheehan-Rooney et al. 2010). This is why it would be of great interest to study the expression of this gene and the functionality of this protein in agnates, in order to know its functional implication in these jawless vertebrate models.
Data availability
The raw data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- ac:
-
Anterior commissure
- A.r:
-
Acipenser ruthenus
- Amy:
-
Amygdaloid complex
- BST:
-
Bed nucleus of the stria terminalis
- Cb:
-
Cerebellum
- cCb:
-
Crista cerebellaris
- Cg:
-
Central gray
- chp:
-
Choroid plexuses
- D.r:
-
Danio rerio
- Dm:
-
Medial zone of the dorsal telencephalic area
- DP:
-
Dorsal pallium
- E.c:
-
Erpetoichthys calabaricus
- fb:
-
Forebrain bundle
- flm:
-
Medial longitudinal fascicle
- fr:
-
Fasciculus retroflexus
- gl:
-
Glomerular layer of the olfactory bulb
- igl:
-
Internal granular layer of the olfactory bulb
- Ip:
-
Interpeduncular nucleus
- Ipn:
-
Interpeduncular neuropil
- L.o:
-
Lepisosteus oculatus
- Lc:
-
Locus coeruleus
- LP:
-
Lateral pallium
- Ma:
-
Mamillary hypothalamic area
- MeA:
-
Medial amygdala
- MP:
-
Medial pallium
- Nsol:
-
Nucleus of the solitary tract
- ob:
-
Olfactory bulb
- oc:
-
Optic chiasm
- OT:
-
Optic tectum
- P.d:
-
Protopterus dolloi
- P.s:
-
Polypterus senegalus
- p1–p3:
-
Diencephalic prosomeres 1–3
- pA:
-
Pallial amygdala
- Pa:
-
Paraventricular hypothalamic area
- P.d:
-
Protopterus dolloi
- POA:
-
Preoptic region
- POAr:
-
Rostral preoptic area
- PT:
-
Pretectum
- PTh:
-
Prethalamus
- r0:
-
Rhombomere 0
- r1–8:
-
Rhombomeres 1–8
- Ras:
-
Superior raphe nucleus
- Ri:
-
Inferior reticular nucleus
- Rm:
-
Median reticular nucleus
- Rs:
-
Superior reticular nucleus
- RTu:
-
Retrotuberal hypothalamic area
- S:
-
Septum
- sco:
-
Subcommissural organ
- sfgs:
-
Stratum fibrosum et griseum superficiale of the optic tectum
- sgc:
-
Stratum griseum centrale of the optic tectum
- SN:
-
Substantia nigra
- sol:
-
Solitary tract
- SP:
-
Subpallium
- SPa:
-
Subparaventricular hypothalamic area
- SPar:
-
Rostral subparaventricular hypothalamic area
- spv:
-
Stratum periventriculare of the optic tectum
- Str:
-
Striatum
- Tegm:
-
Mesencephalic tegmentum
- Th:
-
Thalamus
- Tl:
-
Torus longitudinalis
- TP:
-
Posterior tubercle
- Ts:
-
Torus semicircularis
- Tu:
-
Tuberal hypothalamic area
- v:
-
Ventricle
- vCb:
-
Cerebellar valvula
- Vd:
-
Dorsal nucleus of the ventral telencephalic area
- Vl:
-
Lateral part of the ventral telencephalic area
- Vp:
-
Posterior nucleus of the ventral telencephalic area
- VP:
-
Ventral pallium
- Vs:
-
Supracommissural nucleus of the ventral telencephalic area
- Vv:
-
Ventral nucleus of the ventral telencephalic area
- Zip:
-
Periventricular nucleus of the zona incerta
- VIIIv:
-
Ventral octavolateral area
References
Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, Michaud J, Simeone A (1999) Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev 13(21):2787–2800. https://doi.org/10.1101/gad.13.21.2787
Ahn HJ, Park Y, Kim S, Park HC, Seo SK, Yeo SY, Geum D (2010) The expression profile and function of Satb2 in zebrafish embryonic development. Mol Cells 30(4):377–382. https://doi.org/10.1007/s10059-010-0128-6
Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Fariñas I, Grosschedl R, McConnell SK (2008) Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57(3):364–377. https://doi.org/10.1016/j.neuron.2007.12.012
Alvarez JD, Yasui DH, Niida H, Joh T, Loh DY, Kohwi-Shigematsu T (2000) The MAR-binding protein SATB1 orchestrates temporal and spatial expression of multiple genes during T-cell development. Genes Dev 14(5):521–535
Balamotis MA, Tamberg N, Woo YJ, Li J, Davy B, Kohwi-Shigematsu T, Kohwi Y (2012) Satb1 ablation alters temporal expression of immediate early genes and reduces dendritic spine density during postnatal brain development. Mol Cell Biol 32(2):333–347. https://doi.org/10.1128/MCB.05917-11
Bardet SM, Martinez-de-la-Torre M, Northcutt RG, Rubenstein JL, Puelles L (2007) Conserved pattern of OTP-positive cells in the paraventricular nucleus and other hypothalamic sites of tetrapods. Brain Res Bull 75(2–4):231–235. https://doi.org/10.1016/j.brainresbull.2007.10.037
Bedont JL, Newman EA, Blackshaw S (2015) Patterning, specification, and differentiation in the developing hypothalamus. Wiley Interdiscip Rev Dev Biol 4(5):445–468. https://doi.org/10.1002/wdev.187
Betancur-R R, Broughton RE, Wiley EO et al (2013) The tree of life and a new classification of bony fishes. PLoS Curr. https://doi.org/10.1371/currents.tol.53ba26640df0ccaee75bb165c8c26288
Blackshaw S, Scholpp S, Placzek M, Ingraham H, Simerly R, Shimogori T (2010) Molecular pathways controlling development of thalamus and hypothalamus: from neural specification to circuit formation. J Neurosci 30(45):14925–14930. https://doi.org/10.1523/JNEUROSCI.4499-10.2010
Bouret SG, Simerly RB (2006) Developmental programming of hypothalamic feeding circuits. Clin Genet 70(4):295–301. https://doi.org/10.1111/j.1399-0004.2006.00684.x
Briscoe SD, Ragsdale CW (2018) Molecular anatomy of the alligator dorsal telencephalon. J Comp Neurol 526(10):1613–1646. https://doi.org/10.1002/cne.24427
Britanova O, Akopov S, Lukyanov S, Gruss P, Tarabykin V (2005) Novel transcription factor Satb2 interacts with matrix attachment region DNA elements in a tissue-specific manner and demonstrates cell-type-dependent expression in the developing mouse CNS. Eur J Neurosci 21(3):658–668. https://doi.org/10.1111/j.1460-9568.2005.03897.x
Britanova O, Depew MJ, Schwark M, Thomas BL, Miletich I, Sharpe P, Tarabykin V (2006) Satb2 haploinsufficiency phenocopies 2q32-q33 deletions, whereas loss suggests a fundamental role in the coordination of jaw development. Am J Hum Genet 79(4):668–678. https://doi.org/10.1086/508214
Britanova O, de Juan RC, Cheung A et al (2008) Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57(3):378–392. https://doi.org/10.1016/j.neuron.2007.12.028
Broughton RE, Betancur-R R, Li C, Arratia G, Ortí G (2013) Multi-locus phylogenetic analysis reveals the pattern and tempo of bony fish evolution. PLoS Curr. https://doi.org/10.1371/currents.tol.2ca8041495ffafd0c92756e75247483e
Cai S, Han HJ, Kohwi-Shigematsu T (2003) Tissue-specific nuclear architecture and gene expression regulated by SATB1. Nat Genet 34(1):42–51. https://doi.org/10.1038/ng1146
Chang I, Parrilla M (2016) Expression patterns of homeobox genes in the mouse vomeronasal organ at postnatal stages. Gene Expr Patterns 21(2):69–80. https://doi.org/10.1016/j.gep.2016.08.001
Ciejek EM, Tsai MJ, O’Malley BW (1983) Actively transcribed genes are associated with the nuclear matrix. Nature 306(5943):607–609. https://doi.org/10.1038/306607a0
Close J, Xu H, De Marco García N, Batista-Brito R, Rossignol E, Rudy B, Fishell G (2012) Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci 32(49):17690–17705. https://doi.org/10.1523/JNEUROSCI.3583-12.2012
Denaxa M, Kalaitzidou M, Garefalaki A, Achimastou A, Lasrado R, Maes T, Pachnis V (2012) Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Rep 2(5):1351–1362. https://doi.org/10.1016/j.celrep.2012.10.003
Dickinson LA, Joh T, Kohwi Y, Kohwi-Shigematsu T (1992) A tissue-specific MAR/SAR DNA-binding protein with unusual binding site recognition. Cell 70(4):631–645. https://doi.org/10.1016/0092-8674(92)90432-c
Dickinson LA, Dickinson CD, Kohwi-Shigematsu T (1997) An atypical homeodomain in SATB1 promotes specific recognition of the key structural element in a matrix attachment region. J Biol Chem 272(17):11463–11470. https://doi.org/10.1074/jbc.272.17.11463
Dobreva G, Dambacher J, Grosschedl R (2003) SUMO modification of a novel MAR-binding protein, SATB2, modulates immunoglobulin mu gene expression. Genes Dev 17(24):3048–3061. https://doi.org/10.1101/gad.1153003
Dobreva G, Chahrour M, Dautzenberg M et al (2006) SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell 125(5):971–986. https://doi.org/10.1016/j.cell.2006.05.012
Domínguez L, Morona R, González A, Moreno N (2013) Characterization of the hypothalamus of Xenopus laevis during development. I. The alar regions. J Comp Neurol 521(4):725–759. https://doi.org/10.1002/cne.23222
Domínguez L, González A, Moreno N (2014) Characterization of the hypothalamus of Xenopus laevis during development. II. The basal regions. J Comp Neurol 522(5):1102–1131. https://doi.org/10.1002/cne.23471
Domínguez L, González A, Moreno N (2015) Patterns of hypothalamic regionalization in amphibians and reptiles: common traits revealed by a genoarchitectonic approach. Front Neuroanat 9:3. https://doi.org/10.3389/fnana.2015.00003
Du K, Stöck M, Kneitz S et al (2020) The sterlet sturgeon genome sequence and the mechanisms of segmental rediploidization. Nat Ecol Evol 4(6):841–852. https://doi.org/10.1038/s41559-020-1166-x
Ferran JL, Puelles L, Rubenstein JL (2015) Molecular codes defining rostrocaudal domains in the embryonic mouse hypothalamus. Front Neuroanat 9:46. https://doi.org/10.3389/fnana.2015.00046
Finger TE (2009) Evolution of gustatory reflex systems in the brainstems of fishes. Integr Zool 4(1):53–63. https://doi.org/10.1111/j.1749-4877.2008.00135.x
FitzPatrick DR, Carr IM, McLaren L et al (2003) Identification of SATB2 as the cleft palate gene on 2q32-q33. Hum Mol Genet 12(19):2491–2501. https://doi.org/10.1093/hmg/ddg248
Fu O, Iwai Y, Kondoh K, Misaka T, Minokoshi Y, Nakajima KI (2019) SatB2-expressing neurons in the parabrachial nucleus encode sweet taste. Cell Rep 27(6):1650-1656.e4. https://doi.org/10.1016/j.celrep.2019.04.040
García-Moreno F, Anderton E, Jankowska M, Begbie J, Encinas JM, Irimia M, Molnár Z (2018) Absence of tangentially migrating glutamatergic neurons in the developing avian brain. Cell Rep 22(1):96–109. https://doi.org/10.1016/j.celrep.2017.12.032
Glendining KA, Fisher LC, Jasoni CL (2020) Maternal obesity modulates expression of Satb2 in hypothalamic VMN of female offspring. Life (basel) 10(4):48. https://doi.org/10.3390/life10040048
González A, Northcutt RG (2009) An immunohistochemical approach to lungfish telencephalic organization. Brain Behav Evol 74(1):43–55. https://doi.org/10.1159/000229012
González A, Morona R, López JM, Moreno N, Northcutt RG (2010) Lungfishes, like tetrapods, possess a vomeronasal system. Front Neuroanat 4:130. https://doi.org/10.3389/fnana.2010.00130
González A, Morona R, Moreno N, Bandín S, López JM (2014) Identification of striatal and pallidal regions in the subpallium of anamniotes. Brain Behav Evol 83(2):93–103. https://doi.org/10.1159/000357754
Grady F, Peltekian L, Iverson G, Geerling JC (2020) Direct parabrachial-cortical connectivity. Cereb Cortex 30(9):4811–4833. https://doi.org/10.1093/cercor/bhaa072
Huang Y, Zhang L, Song NN, Hu ZL, Chen JY, Ding YQ (2011) Distribution of Satb1 in the central nervous system of adult mice. Neurosci Res 71(1):12–21. https://doi.org/10.1016/j.neures.2011.05.015
Huang Y, Song NN, Lan W, Hu L, Su CJ, Ding YQ, Zhang L (2013) Expression of transcription factor Satb2 in adult mouse brain. Anat Rec (hoboken) 296(3):452–461. https://doi.org/10.1002/ar.22656
Hughes LC, Ortí G, Huang Y et al (2018) Comprehensive phylogeny of ray-finned fishes (Actinopterygii) based on transcriptomic and genomic data. Proc Natl Acad Sci USA 115(24):6249–6254. https://doi.org/10.1073/pnas.1719358115
Jarvie BC, Chen JY, King HO, Palmiter RD (2021) Satb2 neurons in the parabrachial nucleus mediate taste perception. Nat Commun 12(1):224. https://doi.org/10.1038/s41467-020-20100-8
Jiménez S, López JM, Lozano D, Morona R, González A, Moreno N (2020) Analysis of pallial/cortical interneurons in key vertebrate models of Testudines, Anurans and Polypteriform fishes. Brain Struct Funct 225(7):2239–2269. https://doi.org/10.1007/s00429-020-02123-5
Karthik S, Huang D, Delgado Y et al (2022) Molecular ontology of the parabrachial nucleus. J Comp Neurol 530(10):1658–1699. https://doi.org/10.1002/cne.25307
Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA (2007) The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci 27(50):13624–13634. https://doi.org/10.1523/JNEUROSCI.2858-07.2007
Lee B, Kim J, An T et al (2018) Dlx1/2 and Otp coordinate the production of hypothalamic GHRH- and AgRP-neurons. Nat Commun 9(1):2026. https://doi.org/10.1038/s41467-018-04377-4
Leone DP, Heavner WE, Ferenczi EA, Dobreva G, Huguenard JR, Grosschedl R, McConnell SK (2015) Satb2 regulates the differentiation of both callosal and subcerebral projection neurons in the developing cerebral cortex. Cereb Cortex 25(10):3406–3419. https://doi.org/10.1093/cercor/bhu156
López JM, Lozano D, Morona R, González A (2016) Organization of the nitrergic neuronal system in the primitive bony fishes Polypterus senegalus and Erpetoichthys calabaricus (Actinopterygii: Cladistia). J Comp Neurol 524(9):1770–1804. https://doi.org/10.1002/cne.23922
López JM, Lozano D, Morona R, González A (2019) Organization of the catecholaminergic systems in two basal actinopterygian fishes, Polypterus senegalus and Erpetoichthys calabaricus (Actinopterygii: Cladistia). J Comp Neurol 527(2):437–461. https://doi.org/10.1002/cne.24548
López JM, Jiménez S, Morona R, Lozano D, Moreno N (2022) Analysis of Islet-1, Nkx2.1 Pax6, and Orthopedia in the forebrain of the sturgeon Acipenser ruthenus identifies conserved prosomeric characteristics. J Comp Neurol 530(5):834–855. https://doi.org/10.1002/cne.25249
Lozano D, Morona R, González A, López JM (2019) Comparative analysis of the organization of the catecholaminergic systems in the brain of holostean fishes (Actinopterygii/Neopterygii). Brain Behav Evol 93(4):206–235. https://doi.org/10.1159/000503769
Martins M, Galfrè S, Terrigno M et al (2021) A eutherian-specific microRNA controls the translation of Satb2 in a model of cortical differentiation. Stem Cell Reports 16(6):1496–1509. https://doi.org/10.1016/j.stemcr.2021.04.020
McKenna WL, Ortiz-Londono CF, Mathew TK, Hoang K, Katzman S, Chen B (2015) Mutual regulation between Satb2 and Fezf2 promotes subcerebral projection neuron identity in the developing cerebral cortex. Proc Natl Acad Sci USA 112(37):11702–11707. https://doi.org/10.1073/pnas.1504144112
Moreno N, González A (2003) Hodological characterization of the medial amygdala in anuran amphibians. J Comp Neurol 466(3):389–408. https://doi.org/10.1002/cne.10887
Moreno N, González A (2005) Central amygdala in anuran amphibians: neurochemical organization and connectivity. J Comp Neurol 489(1):69–91. https://doi.org/10.1002/cne.20611
Moreno N, Domínguez L, Morona R, González A (2012) Subdivisions of the turtle Pseudemys scripta hypothalamus based on the expression of regulatory genes and neuronal markers. J Comp Neurol 520(3):453–478. https://doi.org/10.1002/cne.22762
Nielsen JV, Blom JB, Noraberg J, Jensen NA (2010) Zbtb20-induced CA1 pyramidal neuron development and area enlargement in the cerebral midline cortex of mice. Cereb Cortex 20(8):1904–1914. https://doi.org/10.1093/cercor/bhp261
Nomura T, Yamashita W, Gotoh H, Ono K (2018) Species-specific mechanisms of neuron subtype specification reveal evolutionary plasticity of amniote brain development. Cell Rep 22(12):3142–3151. https://doi.org/10.1016/j.celrep.2018.02.086
Northcutt RG, Westhoff G (2011) Connections of the medial telencephalic wall in the spotted African Lungfish. Brain Behav Evol 77(1):14–32. https://doi.org/10.1159/000322549
Otani T, Marchetto MC, Gage FH, Simons BD, Livesey FJ (2016) 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell 18(4):467–480. https://doi.org/10.1016/j.stem.2016.03.003
Paolino A, Fenlon LR, Suárez R, Richards LJ (2018) Transcriptional control of long-range cortical projections. Curr Opin Neurobiol 53:57–65. https://doi.org/10.1016/j.conb.2018.05.005
Paolino A, Fenlon LR, Kozulin P, Haines E, Lim JWC, Richards LJ, Suárez R (2020) Differential timing of a conserved transcriptional network underlies divergent cortical projection routes across mammalian brain evolution. Proc Natl Acad Sci USA 117(19):10554–10564. https://doi.org/10.1073/pnas.1922422117
Porter BA, Mueller T (2020) The zebrafish amygdaloid complex—functional ground plan, molecular delineation, and everted topology. Front Neurosci 14:608. https://doi.org/10.3389/fnins.2020.00608
Pradhan SJ, Reddy PC, Smutny M et al (2021) Satb2 acts as a gatekeeper for major developmental transitions during early vertebrate embryogenesis. Nat Commun 12(1):6094. https://doi.org/10.1038/s41467-021-26234-7
Puelles L, Rubenstein JL (2003) Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci 26(9):469–476. https://doi.org/10.1016/S0166-2236(03)00234-0
Puelles L, Rubenstein JL (2015) A new scenario of hypothalamic organization: rationale of new hypotheses introduced in the updated prosomeric model. Front Neuroanat 9:27. https://doi.org/10.3389/fnana.2015.00027
Ren J, Isakova A, Friedmann D et al (2019) Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. Elife 8:e49424. https://doi.org/10.7554/eLife.49424
Sheehan-Rooney K, Pálinkášová B, Eberhart JK, Dixon MJ (2010) A cross-species analysis of Satb2 expression suggests deep conservation across vertebrate lineages. Dev Dyn 239(12):3481–3491. https://doi.org/10.1002/dvdy.22483
Suárez R, Gobius I, Richards LJ (2014) Evolution and development of interhemispheric connections in the vertebrate forebrain. Front Hum Neurosci 8:497. https://doi.org/10.3389/fnhum.2014.00497
Suárez R, Paolino A, Fenlon LR, Morcom LR, Kozulin P, Kurniawan ND, Richards LJ (2018) A pan-mammalian map of interhemispheric brain connections predates the evolution of the corpus callosum. Proc Natl Acad Sci USA 115(38):9622–9627. https://doi.org/10.1073/pnas.1808262115
Suzuki IK, Hirata T (2014) A common developmental plan for neocortical gene-expressing neurons in the pallium of the domestic chicken Gallus gallus domesticus and the Chinese softshell turtle Pelodiscus sinensis. Front Neuroanat 8:20. https://doi.org/10.3389/fnana.2014.00020
Tosches MA, Laurent G (2019) Evolution of neuronal identity in the cerebral cortex. Curr Opin Neurobiol 56:199–208. https://doi.org/10.1016/j.conb.2019.04.009
Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, Laurent G (2018) Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360(6391):881–888. https://doi.org/10.1126/science.aar4237
Vaughn JP, Dijkwel PA, Mullenders LH, Hamlin JL (1990) Replication forks are associated with the nuclear matrix. Nucleic Acids Res 18(8):1965–1969. https://doi.org/10.1093/nar/18.8.1965
Witteveen JS, Middelman A, van Hulten JA, Martens GJ, Homberg JR, Kolk SM (2013) Lack of serotonin reuptake during brain development alters rostral raphe-prefrontal network formation. Front Cell Neurosci 7:143. https://doi.org/10.3389/fncel.2013.00143
Yasui D, Miyano M, Cai S, Varga-Weisz P, Kohwi-Shigematsu T (2002) SATB1 targets chromatin remodelling to regulate genes over long distances. Nature 419(6907):641–645. https://doi.org/10.1038/nature01084
Zarate YA, Kaylor J, Fish J (2017) SATB2 Associated Syndrome. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews. Seattle (WA): University of Washington, Seattle; 1993–2023
Zarate YA, Bosanko KA, Caffrey AR, Bernstein JA, Martin DM, Williams MS, Berry-Kravis EM, Mark PR, Manning MA, Bhambhani V, Vargas M, Seeley AH, Estrada-Veras JI, van Dooren MF, Schwab M, Vanderver A, Melis D, Alsadah A, Sadler L, Van Esch H, Callewaert B, Oostra A, Maclean J, Dentici ML, Orlando V, Lipson M, Sparagana SP, Maarup TJ, Alsters SI, Brautbar A, Kovitch E, Naidu S, Lees M, Smith DM, Turner L, Raggio V, Spangenberg L, Garcia-Miñaúr S, Roeder ER, Littlejohn RO, Grange D, Pfotenhauer J, Jones MC, Balasubramanian M, Martinez-Monseny A, Blok LS, Gavrilova R, Fish JL (2019) Mutation update for the SATB2 gene. Hum Mutat 40(8):1013–1029. https://doi.org/10.1002/humu.23771
Zhang L, Song NN, Chen JY, Huang Y, Li H, Ding YQ (2012) Satb2 is required for dendritic arborization and soma spacing in mouse cerebral cortex. Cereb Cortex 22(7):1510–1519. https://doi.org/10.1093/cercor/bhr215
Zhang Q, Zhang L, Huang Y, Ma P, Mao B, Ding YQ, Song NN (2021) Satb2 regulates the development of dopaminergic neurons in the arcuate nucleus by Dlx1. Cell Death Dis 12(10):879. https://doi.org/10.1038/s41419-021-04175-9
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by the Spanish Ministry of Science and Innovation (grant no. PID2020-112681GB100), and the Santander/Complutense University of Madrid, Grant/Award Number: PR108/20-17.
Author information
Authors and Affiliations
Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. NM devised the study. DL, JML, VR and AM performed the experiments. SJ performed the sequence analysis. JML and NM analyzed the neuroanatomical results. RM, SJ and DL led the figure preparation, edited by NM. NM wrote most of the article, corrected and edited by JML, and DL. All authors approved the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The original research reported herein was performed according to the regulations and laws established by European Union (2010/63/EU) and Spain (Royal Decree 118/2021) for care and handling of animals in research and after approval from the Complutense University to conduct the experiments described. Reference number from the Complutense Animal Ethics Committee: ES-28079-0000086.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lozano, D., López, J.M., Jiménez, S. et al. Expression of SATB1 and SATB2 in the brain of bony fishes: what fish reveal about evolution. Brain Struct Funct 228, 921–945 (2023). https://doi.org/10.1007/s00429-023-02632-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-023-02632-z