Abstract
Transcranial direct current stimulation (tDCS) applied to the primary motor cortex (M1), and cerebellum (CB) can change the level of M1 corticospinal excitability (CSE). A randomized double-blinded crossover, the sham-controlled study design was used to investigate the effects of concurrent bilateral anodal tDCS of M1 and CB (concurrent bilateral a-tDCSM1+CB) on the CSE. Twenty-one healthy participants were recruited in this study. Each participant received anodal-tDCS (a-tDCS) of 2 mA, 20 min in four pseudo-randomized, counterbalanced sessions, separated by at least 7 days (7.11 days ± 0.65). These sessions were bilateral M1 stimulation (bilateral a-tDCSM1), bilateral cerebellar stimulation (bilateral a-tDCSCB), concurrent bilateral a-tDCSM1+CB, and sham stimulation (bilateral a-tDCSSham). Transcranial magnetic stimulation (TMS) was delivered over the left M1, and motor evoked potentials (MEPs) of a contralateral hand muscle were recorded before and immediately after the intervention to measure CSE changes. Short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), and long interval intracortical inhibition (LICI) were assessed with paired-pulse TMS protocols. Anodal-tDCS significantly increased CSE after concurrent bilateral a-tDCSM1+CB and bilateral a-tDCSCB. Interestingly, CSE was decreased after bilateral a-tDCSM1. Respective alterations in SICI, LICI, and ICF were seen, including increased SICI and decreased ICF, which indicate the involvement of glutamatergic and GABAergic systems in these effects. These results confirm that the concurrent bilateral a-tDCSM1+CB have a facilitatory effect on CSE, whereas bilateral a-tDCSM1 exert some inhibitory effects. Moreover, the effects of the 2 mA, 20 min a-tDCS on the CB were consistent with its effects on the M1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcranial direct current stimulation (tDCS) is a technique of applying a weak direct current (0.5–2 mA) for a relatively long period (usually less than 30 min) to the scalp via two or more surface electrodes. The effects of tDCS are mainly induced by modulation of spontaneous neuronal activity (Nitsche and Paulus 2000; Nitsche et al. 2005; Rossini et al. 2015) and lead to neuroplastic (Karabanov et al. 2015; Cirillo et al. 2017; Huang et al. 2017) and corticospinal excitability (CSE) changes (Nitsche and Paulus 2000; Nitsche et al. 2005; Soekadar et al. 2014; Marquez et al. 2015; Rossini et al. 2015). Over the last two decades, tDCS has gained popularity due to its noninvasive nature, simplicity of use and effects on neuronal activity (Nitsche 2011; Soekadar et al. 2014; Rossini et al. 2015; Vaseghi et al. 2015; Dissanayaka et al. 2017; Morya et al. 2019). Previously it was suggested that anodal tDCS (a-tDCS) acts as a facilitatory technique, increasing spontaneous neuronal activity and CSE, while cathodal tDCS acts as an inhibitory technique, reducing the spontaneous neuronal activity and CSE. However, emerging evidence suggests that the conventional notion about the polarity-dependent effects of tDCS is not always the same (Monte-Silva et al. 2013; Hassanzahraee et al. 2020). This can be explained by metaplastic mechanisms responsible for a-tDCS effects. In addition, these studies suggest that many variables can affect the a-tDCS outcomes, such as duration threshold (Monte-Silva et al. 2013; Hassanzahraee et al. 2020), other than just its polarity. It has been demonstrated the type of plasticity induced can be changed by periodical stimulation, and a specific time window is critical for its induction (Monte-Silva et al. 2013). Therefore, determining the a-tDCS effect is not as straightforward as it seems.
Apart from cortical behaviors, other outcomes were also the interest in tDCS field, such as cognition (Hill et al. 2016; Verissimo et al. 2016; Katsoulaki et al. 2017; Martin et al. 2018), motor learning (Karok and Witney 2013; Ammann et al. 2016; Wiltshire and Watkins 2020; Wang et al. 2021) and postural balance in humans (Kaminski et al. 2013; Ehsani et al. 2017; Saruco et al. 2017, 2018; Manor et al. 2018; Nomura and Kirimoto 2018). However, interestingly, despite the significant functional and structural connection between M1 and cerebellum shown by neuroimaging and transcranial magnetic stimulation (TMS) studies (Bestmann et al. 2004; Jung et al. 2020; Peters et al. 2020; Spampinato et al. 2020), the majority of tDCS studies that targeted these two brain regions utilized single-site stimulation of either of these sites and mainly unilateral to improve related outcomes, such as CSE (Marquez et al. 2015; Dedoncker et al. 2016; Behrangrad et al. 2019), motor control activities, such as balance (Bellebaum and Daum 2007; Kaminski et al. 2016, 2017; Baharlouei et al. 2020), and motor learning (Karok and Witney 2013; Ammann et al. 2016; Wiltshire and Watkins 2020; Wang et al. 2021), etc. Although some found promising results (Steiner et al. 2016; Saruco et al. 2017, 2018; Poortvliet et al. 2018, Baharlouei et al. 2020), some could not find any significant changes (Horvath et al. 2015; Craig and Doumas 2017; Ehsani et al. 2017; Kaminski et al. 2017; Medina and Cason 2017; Pohjola et al. 2017; Steiner et al. 2020; Wiltshire and Watkins 2020; Wang et al. 2021).
This discrepancy can be explained by the structural and functional connectivity of bilateral M1 and bilateral cerebellum that is more noticeable in activities requiring higher degrees of motor control, and activity of both sides of the body, such as postural balance (Bostan et al. 2013; Carrillo et al. 2013; Ishikawa et al. 2016; Spampinato et al. 2020). The cerebellum receives information from different brain areas in the frontal, parietal, temporal, and occipital lobes and funnel them back to M1 through the ventrolateral nuclei of the thalamus (Allen and Tsukahara 1974; Bellebaum and Daum 2007; Bostan et al. 2013). Thus, these cerebello-cortical pathways can be defined as ways of collecting information from widespread areas of the cerebral cortex to help with the smooth execution of each movement through the M1 area (Allen and Tsukahara 1974; Bellebaum and Daum 2007; Bostan et al. 2013). These findings suggest that more exploratory studies are necessary to refine the conventional single-site unilateral a-tDCS technique and introduce a novel tDCS approach that stimulates multiple brain areas concurrently.
Recently, some neuroscience and neurorehabilitation studies suggested an innovative optimization technique, dual-site stimulation, and concluded superiority of this technique over the conventional single-site stimulation (Vaseghi et al. 2015; Hill et al. 2018; Chen et al. 2019; Koshy et al. 2020). Dual-site stimulation is a technique that is theoretically referred to as concurrent stimulation of two functionally related brain sites within the same hemisphere or across opposite hemispheres (Vaseghi et al. 2015; Hill et al. 2018; Chen et al. 2019; Koshy et al. 2020). Furthermore, although many studies are providing significant structural and functional connection between the bilateral M1 and cerebellum in motor control activities, all of the studies have only investigated the effects of unilateral M1 or cerebellum on CSE and cortico-cortical excitability (Dissanayaka et al. 2017; Behrangrad et al. 2019). No study has explored the effect of the dual-site stimulation of bilateral M1 and cerebellum on the CSE and possible neurophysiological mechanisms behind it by assessing cortico-cortical excitability.
Therefore, to establish the limitations of the single-site stimulation and to propose a new framework for future studies, concurrent bilateral dual-site a-tDCS of cerebellum and M1 is utilized in this study. Since the tDCS can modulate the spontaneous firing rate without causing any action potential (Gandiga et al. 2006), it is expected that the excitatory and/or inhibitory postsynaptic potentials induced by dual-site a-tDCS may modulate the neuronal excitability more efficiently than conventional single site a-tDCS. As a proof of concept study, due to the novelty of the proposed approach, this study is investigating how dual-site a-tDCS will affect CSE and the mechanisms underlying these changes. The aims of this study are:
-
1.
To investigate the effects of bilateral a-tDCS of M1 (a-tDCSM1) on CSE.
-
2.
To investigate the effects of bilateral a-tDCS of cerebellum (a-tDCSCB) on CSE.
-
3.
To investigate the effects of concurrent bilateral a-tDCS of M1 and cerebellum (a-tDCSM1+CB) on CSE.
-
4.
To investigate the underlying mechanisms behind the changes on CSE.
-
5.
To compare the effects of bilateral a-tDCSM1, bilateral a-tDCSCB, concurrent bilateral a-tDCSM1+CB on CSE and cortico-cortical excitability.
Materials and methods
Participants
Twenty-one healthy non-smoking volunteers (10 females, 11 males; mean age 23.66 years ± 4.53) were recruited in this study using a simple non-probability sampling method. The sample size was calculated based on the critical effect size generated from a pilot study on eight participants (power of 0.8, α = 0.05, effect size = 0.9). All participants were right-handed, determined by the Edinburgh Handedness Inventory (58.23 ± 8.8) (Oldfield 1971). Participants were included from a pool of young, healthy, non-smoking adults aged 18–35 years.
Exclusion criteria included any history of neurological, rheumatoid, or musculoskeletal disorders, intracranial metal implantation, implanted devices, such as cardiac pacemakers, cochlear implants, medical pumps, or intracardiac lines, consuming medications for any neurological condition (Wassermann 1998; Brunoni et al. 2011). In addition, participants were asked not to consume any alcohol or caffeine 24 h before the experimental sessions and sleep at least 7 h the night before each session. The experimental protocol was performed in accordance with the Declaration of Helsinki and approved by the Human Research Ethics Committee, Monash University, Melbourne, Australia. Informed consent was obtained from all participants included in the study.
Study design
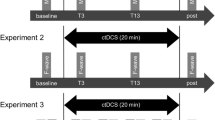
A randomized double-blinded crossover, sham-controlled study design was used in this study. The design involves participation in four experimental conditions (Fig. 1) in a random order: 1. bilateral a-tDCS of M1 (a-tDCSM1), 2. bilateral a-tDCS of cerebellum (a-tDCSCB), 3. concurrent bilateral a-tDCSM1+CB, and 4. bilateral sham a-tDCS (a-tDCSSham).
Schematic representation of the experimental procedure for each session. The timeline shows the order of the procedures from left to right. TMS transcranial magnetic stimulation, S session, MEPs motor evoked potentials, CSE corticospinal excitability, ICF intra-cortical facilitation, LICI long intra-cortical inhibition, SICI short intra-cortical inhibition, A-tDCS anodal–transcranial direct current stimulation, RMT resting motor threshold, TS 1 mV test stimulus intensity required for peak-to-peak MEP amplitude of approximately 1 mV, Tpre baseline, T0 Immediately after the intervention
All participants attended all four experimental sessions, pseudo-randomly in a counterbalanced manner, separated by at least 7 days (7.11 days ± 0.65) (Boggio et al. 2007). Moreover, to reduce the risk of circadian influences, each participant was examined at the same time of the day for all experimental sessions (Krause and Cohen Kadosh 2014; Li et al. 2015). All participants were blinded to the stimulation conditions and the purpose of this study. The participants were unaware of the allocated session, and the blinding integrity was checked after completion of each session by asking about the nature, active or sham, of the stimulation they had received. Two researchers were involved in the present study, one as the a-tDCS administrator and the other as the assessor of the outcome measures. The administrator that was responsible for delivering a-tDCS interventions was not involved in any data collection or analysis. The assessor, who was responsible for data collection and analysis, was blinded to all experimental conditions and the allocation. All participants received a-tDCS under each of the four different experimental conditions.
Experimental procedures
Electromyography
Participants were seated upright in an adjustable chair with the right forearm and the wrist in a pronated and neutral position, resting on a pillow. A standard skin preparation procedure (alcohol cleaning and abrading) of each electrode placement site was done to provide proper surface contact and reduce skin resistance (Gilmore and Meyers 1983). Surface electromyography (EMG) was recorded from the first dorsal interosseous (FDI) at rest using pre-gelled self-adhesive bipolar Ag/AgCl disposable surface electrodes with 2 cm inter-electrode distance (measured from the centers of the electrodes). The location of FDI was determined based on anatomical landmarks, palpation, and voluntary muscle contraction through manually resisted index finger abduction. A ground electrode was placed over the ipsilateral right ulnar styloid process. The EMG raw signals were bandpass filtered (10–500 Hz), amplified by 1000 (1000×), sampled at 1000 Hz. The data were collected on PC software (LabChart™ software, AD Instruments, Australia) via a laboratory analogue–digital interface (PowerLab, AD Instruments, Australia) and stored for later offline analysis.
Tools for assessment of CSE and intracortical excitability
Single- and paired-pulse magnetic stimuli were delivered by a 70 mm figure of eight magnetic coil (Magstim Company Limited, UK), connected to a MagPro R30 stimulator (Mag Venture, Denmark). The coil was placed over the left M1 for FDI muscle, angled 45° from the midline sagittal plane and tangential to the scalp to ensure that the induced current flowed in a posterior–anterior direction (Rossini and Rossi 1998; Schmidt et al. 2009). The area of stimulation with the largest MEP responses was defined as the "hotspot". This spot was marked on the scalp to maximize the consistency of coil placement throughout the entire experiment. The parameter estimation by sequential testing (PEST) technique was used to determine the resting motor threshold (RMT) (Awiszus 2003; Dissanayaka et al. 2018). The RMT was determined based on the International Federation of Clinical Neurophysiology guidelines (Ziemann et al. 1996; Ilic et al. 2002; Rossini et al. 2015). The RMT was defined as the lowest stimulus intensity to elicit the MEP with a peak-to-peak amplitude of 0.05 mV or more in 3 out of 6 consecutive stimuli in the resting FDI (Devanne et al. 2006; Li et al. 2015). The TMS intensity, defined as a percentage of maximum stimulator output (%MSO), was adjusted to elicit a mean MEP amplitude of about 1 mV peak-to-peak (SI 1 mV) in the resting FDI (Nitsche and Paulus 2000, 2001; Rossini et al. 2015). In this study, the baseline MEP means within the range of 1 mV ± 20% were accepted (Labruna et al. 2016). Both single and paired TMS pulse was utilized based on standard protocols to calculate CSE, Short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), and long interval intracortical inhibition (LICI) (Ziemann et al. 1996; Ilic et al. 2002; Shirota et al. 2010). All TMS assessments were carried out by the same assessor (SHB), who was trained for reliable use of TMS.
Single-pulse TMS: assessment of corticospinal excitability
Twenty-five single-pulse stimuli with 5-s inter-pulse intervals were delivered, and 25 consecutive elicited MEPs were recorded from the right FDI muscle. The average peak-to-amplitudes of 25 MEPs were calculated before (Tpre) and immediately (T0) after applying a-tDCS to evaluate the tDCS-induced changes on CSE. The baseline TMS intensity was adjusted to elicit 1 mV peak-to-peak MEP amplitude (Thush study) and is kept constant during post-intervention assessments. It is worth mentioning that no significant differences were found between these two TMS intensities (p = 0.4, Cohen’s d = − 0.16, CI = − 0.76 to 0.45).
Paired-pulse paradigm: assessment of intracortical inhibition and facilitation
The same SI 1 mV applied for a single pulse was utilized as a test stimulus for paired pulses, preceded by a suprathreshold or subthreshold conditioning stimulus. Paired-pulse TMS can provide important information about the intracortical inhibitory (SICI and LICI) and excitatory (ICF) neural circuits (Chen et al. 2008; Vucic et al. 2013). In the current study, SICI, LICI, and ICF were measured by paired-pulse TMS (Valls-Sole et al. 1992; Kujirai et al. 1993). In SICI and ICF, a subthreshold conditioning stimulus (80% of RMT) is followed by a suprathreshold test stimulus, SI 1 mV, with an inter-stimulus interval (ISI) of 3 and 10 ms, respectively. In LICI, a suprathreshold conditioning stimulus applied 150 ms prior to the test stimulus (motor threshold of 1mv) (Kujirai et al. 1993). The SICI, LICI, and ICF were calculated using the peak-to-peak amplitude for each elicited MEPs. The size of the conditioned MEP was expressed as a percentage of the unconditioned test MEP to assess the modulations of SICI, LICI and ICF. The test stimulus intensity was adjusted to achieve a baseline MEP of about 1 mV (0.8–1.3 mV) and readjusted the intensity for the paired-pulse recordings after the application of a-tDCS to compensate for the effects of the intervention on the MEP amplitude (Nitsche et al. 2005; Pellegrini et al. 2021a, b).
Transcranial direct current stimulation
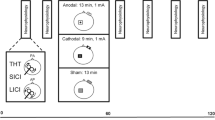
Anodal-tDCS was delivered using a battery-driven direct current stimulator (NeuroConn, Germany) through a pair of saline-soaked surface sponge electrodes. (active, 3 cm × 9 cm, current density: 0.083 mA/cm2); return, 5 cm × 7 cm, current density: 0.057 mA/cm2) (Fig. 2 shows active electrode placement). For bilateral a-tDCSM1, the active electrode (anode) was centered on the Cz, based on the international 10–20 extended EEG system, to cover M1 for bilateral lower extremity, trunk, and upper extremity muscles, and the return electrode (cathode) was placed over the right supraorbital area. The return electrode was deliberately chosen larger to reduce the current density and, therefore, reduce its potential effects on the anterior pole of the brain (Nitsche and Paulus 2000, 2001). For bilateral a-tDCSCB, the active electrode (anode) was placed centrally 1 cm below the inion of the occipital bone to cover both the right and left cerebellar hemispheres. The return electrode (cathode) was positioned extracephalic on the right deltoid area (Ferrucci et al. 2015; Ehsani et al. 2017). The landmarks for placing active electrodes were identified by measuring and marking the skull before electrode placement, based on the previous studies (Kaminski et al. 2016; Ehsani et al. 2017; Baharlouei et al. 2020). To facilitate the blinding in this study, the electrode montage used for concurrent bilateral a-tDCSM1+CB was also used for bilateral a-tDCSM1, bilateral a-tDCSCB, and bilateral a-tDCSsham. Both M1 and CB channels were turned on for bilateral stimulation, while for single-site bilateral a-tDCSM1 and concurrent bilateral a-tDCSM1+CB, only one of the channels was turned on. For a-tDCSsham, both or one of the channels were turned on but for only 30 s.
The electrodes were fixed with two horizontal and perpendicular elastic straps. Two pairs of electrodes for both M1 and CB stimulation were applied over the designated positions in all experimental conditions. Each pair of electrodes were connected to a separate a-tDCS device. In each experimental session, depends on the experimental condition, one or both devices were turned on. The current intensity was set at 2 mA, and the duration of stimulation was 20 min with a 15 s fade-in at the start and 15 s fade-out at the ends of stimulation to minimize the abrupt changes in current intensity and, therefore, discomfort. In the sham experiment, the a-tDCS was turned off after 30 s (Gandiga et al. 2006) (Fig. 1).
Assessment of the side effects
All participants were asked to answer a questionnaire concerning the side or adverse effects of stimulation in all four experimental conditions at 0–5 min, 6–10 min, 11–15 min, and 16–20 min of the stimulation time. The questionnaire included rating scales for common side effects, such as itching, tingling, burning sensation, or any other side effects under the electrodes (Boggio et al. 2007; George and Aston-Jones 2010; Brunoni et al. 2011). All participants were asked to rate the intensity of each item during and after stimulation based on a numerical analog scale, with 0 representing "no sensation" and 10 representing "the worst sensation imaginable". The items included numbness, itching, burning sensation, pain, fatigue, and headache. In addition, at the end of each experiment, participants were requested to indicate the nature of the stimulation they received (active or sham) by choosing' Yes', 'No', or 'cannot say' as the answer.
Data analysis
Peak-to-peak amplitudes of 25 single-pulse MEPs were automatically calculated online for each time point of measurement, using a custom-designed macro in Powerlab data recording and analysis 8/30 software (ADInstruments, Australia).. The size of the conditioned MEP was calculated as a percentage of the unconditioned test MEPs to calculate SICI, LICI, and ICF. The data with no knowledge of experimental conditions were blindly analyzed by SPSS version 22 (IBM Corp., Armonk, NY, USA). A one-way repeated measure ANOVA (RM-ANOVA) on baseline values in different experimental sessions for all dependent variables (RMT, SI 1 mV, CSE, SICI, LICI, and ICF) was carried to rule out the carry-over effects of experimental conditions. The normal distribution of data for each outcome measure was examined by the Shapiro–Wilk test, and all variables were normally distributed. The effects of two independent variables, i.e., "the experimental conditions" with four levels (M1, cerebellum, dual-site, and sham stimulations) and "time" with two levels (Tpre, T0), on CSE, SICI, LICI, and ICF, were assessed through a two-way repeated measures ANOVA. Mauchly's test was carried out to determine the validity of the sphericity assumption for repeated measures ANOVA. The Greenhouse–Geisser corrected significance values were used when sphericity was lacking (Meyers et al. 2006). When ANOVA showed significant results (p < 0.05), post-hoc comparisons were performed using the Bonferroni correction.
Furthermore, to determine whether participants were effectively blinded to the stimulation condition (active or sham), participants were asked if they could differentiate between stimulation they received after completing each experiment. The Pearson's chi-square test was carried out on rating scales recorded by questionnaire. Moreover, for side effect analysis, a one-way RM-ANOVA was carried out on the mean values of the rating scale recorded to evaluate any significant differences between the participants' feelings during active and sham conditions. The critical level of significance was set to p < 0.05. All results in tables and figures are displayed as means ± standard error measurements (SEM). However, the participant's sensation scores during experimental conditions were reported as means ± standard deviation (SD). In addition, based on the null hypothesis statistical test (significance tests and hypothesis tests), significant statistical testing is not enough to rely on, as it provides information about the existence of the effects (Herbert 2019). Therefore, Cohen's d effect size (Cohen 1992; Greenfield et al. 1997; Hickey et al. 2018) was calculated to estimate the effect size of the included studies. According to the thresholds explained by Cohen, the effect size magnitude was interpreted as small (d = 0.20), moderate (d = 0.50), and large (d = 0.80) (Cohen 1992). In this study, the p value, followed by the Cohen's d effect size and 95% confidence interval (95% CI), is reported in the results.
Results
All 21 healthy participants completed all experimental sessions. The Shapiro–Wilk test showed normality in all data sets. The results of the one-way RM-ANOVA showed no significant difference in baseline values for RMT, SI 1mv, and MEPs (CSE, SICI, LICI, and ICF) at all experimental conditions (Table 1).
The effects of bilateral a-tDCS on CSE
The two-way RM-ANOVA indicated significant main effects of the experimental conditions (F = 4.18, p = 0.009) and interaction of condition and time (F = 8.927, p < 0.0001). However, the results did not reveal any significant main effect of time (F = 0.007, p = 0.933). Figure 2 summarizes the CSE changes in all participants in all four experimental conditions. The post-hoc comparisons with Bonferroni corrections revealed significant difference in MEP amplitude between bilateral a-tDCSM1 and concurrent bilateral a-tDCSM1+CB (p = 0.002, Cohen’s d = 1.67, 95% CI 0.94–2.34), and bilateral a-tDCSM1 and bilateral a-tDCSCB (p < 0.0001, Cohen’s d = 1.6, 95% CI 0.88–2.26) (Fig. 3).
Comparison of the effects of bilateral a-tDCS M1, concurrent bilateral a-tDCSM1+CB, bilateral a-tDCSCB, with bilateral a-tDCSsham on the percentage of changes of the peak-to-peak amplitude of MEPs (A), ICF (conditioned MEP/test MEP × 100) (B), LICI (conditioned MEP/Test MEP × 100) (C), and SICI (conditioned MEP/test MEP × 100) (D). The (*) shows significant differences, p < 0.05. All of the data are normalized, and ratios of the percentage changes have been mentioned. Each dot represents one participant. Data are reported as mean ± SEM. Lines show the means. Error bars indicate SEM
Similarly, the comparison of bilateral a-tDCSsham with a-tDCSM1 (p = 0.033, Cohen’s d = 1.29, 95% CI 0.6–1.92), a-tDCSCB (p = 0.049, Cohen’s d = − 1.24, 95% CI − 1.87 to − 0.56), respectively, and concurrent bilateral a-tDCSM1+CB (p = 0.02, Cohen’s d = 0.92, 95% CI 0.27–1.54). In addition, comparing the peak-to-peak MEP amplitudes of Tpre and T0 provided significant decrease following bilateral a-tDCSM1 (p = 0.01, Cohen’s d = − 1.03, 95% CI − 1.66 to − 0.37). Moreover, significant increase were found following bilateral a-tDCSCB (p = 0.037, Cohen’s d = 0.47, 95% CI − 1.07 to 0.15) and concurrent bilateral a-tDCSM1+CB (p = 0.048, Cohen’s d = 0.26, 95% CI − 0.36 to 0.86). In addition, comparison of the peak-to-peak MEP amplitudes of Tpre and T0 in sham stimulation didn’t show any significant changes (p = 0.35, Cohen’s d = − 0.07, 95% CI − 0.67 to 0.54). Figure 4 summarizes the CSE changes in all participants in each experimental condition.
Effects of a-tDCS on the peak-to-peak amplitude of MEPs (A), ICF (B), LICI (C) and SICI (D) with bilateral a-tDCSM1, concurrent bilateral a-tDCSM1+CB, bilateral a-tDCSCB, and bilateral a-tDCSsham. The (*) shows significant differences, p < 0.05. Each dot represents one participant. Data are reported as mean ± SEM. Lines show the means. Error bars show SEM
The effects of bilateral a-tDCS on SICI
The RM-ANOVA showed a significant effect of condition and time interaction (F = 4.789, p = 0.027). However, there was no significant main effect of condition (F = 3.928, p = 0.103) or time (F = 0.011, p = 0.916). A significant decrease was seen in the SICI level after bilateral a-tDCSM1 application (p = 0.041, Cohen’s d = − 0.47, 95% CI − 1.07 to 0.15) compared to the respective baseline values (Fig. 4). However, no significant changes were seen comparing Tpre and T0 bilateral a-tDCSCB (p = 0.532, Cohen’s d = 0.45, 95% CI − 0.17 to 1.05), and concurrent bilateral a-tDCSM1+CB (p = 0.196, Cohen’s d = − 0.14, 95% CI − 0.74 to 0.47). Moreover, significant changes found between bilateral a-tDCSCB and bilateral a-tDCSSham (p = 0.034, Cohen’s d = − 0.38, 95% CI − 0.98 to 0.24) (Fig. 3). However, no significant differences were found between the sham and other experimental conditions (p a-tDCSM1 = 0.93, Cohen’s d a-tDCSM1 = − 0.5, 95% CI − 1.07 to 0.15) (p a-tDCSM1+CB = 0.05, Cohen’s d a-tDCSM1+CB = 0.19, 95% CI − 0.42 to 0.79) (Fig. 3).
The effects of bilateral a-tDCS on LICI
The RM-ANOVA revealed a significant interaction of stimulation (F = 7.679, p = 0.001) and interaction of condition and time (F = 6.192, p = 0.005). Whereas, there was no significant main effect of time (F = 1.671, p = 0.211). Significant increases were seen in the LICI after bilateral a-tDCSM1 compared to respective baseline values (p = 0.031, Cohen’s d = − 0.66, 95% CI − 1.26 to − 0.02) (Fig. 4). However, no significant changes were seen in the after bilateral a-tDCSCB (p = 0.383, Cohen’s d = − 0.47, 95% CI − 1.08 to 0.15), concurrent bilateral a-tDCSM1+CB (p = 0.166, Cohen’s d = 0.19, 95% CI − 0. 42 to 0.79), and a-tDCSSham (p = 0.509, Cohen’s d = − 0.24, 95% CI − 0.84 to 0.38) as compared to the respective baseline values (Fig. 4). Moreover, no significant changes were seen between experimental conditions compared with a-tDCSSham conditions (p a-tDCSM1 = 0.465, Cohen’s d a-tDCSM1 = − 0.33, 95% CI − 0.93 to 0.28) (p a-tDCSCB = 0.578, Cohen’s d a-tDCSCB = 0.26, 95% CI − 0.35 to 0.86) (p a-tDCSM1+CB = 0.342, Cohen’s d a-tDCSM1+CB = 0.41, 95% CI − 0.21 to 1.02) (Fig. 3).
The effects of bilateral a-tDCS on ICF
The results of RM-ANOVA provided a significant main effect of ‘conditions’ on ICF (F = 7.679, p < 0.001), and a significant interaction of condition and time (F = 6.192, p = 0.005). However, there was no significant main effect of ‘time’ for ICF (F = 1.671, p = 0.211). Pairwise comparisons showed that ICF decreased significantly following bilateral a-tDCSM1 (p = 0.01, Cohen’s d = − 0.96, 95% CI − 1.57 to − 0.3) compared to the baseline (Fig. 4). However, no significant differences were found after bilateral a-tDCSCB (p = 0.13, Cohen’s d = 0.7, 95% CI 0.06–1.3) or concurrent bilateral a-tDCSM1+CB (p = 0.09, Cohen’s d = 0.53, 95% CI − 0.09 to 1.14). In addition, a significant decrease were found in the ICF level after bilateral a-tDCSCB (p ˂ 0.005, Cohen’s d = 1, 95% CI 0.34–1.62) and concurrent bilateral a-tDCSM1+CB (p = 0.013, Cohen’s d = 1.04, 95% CI 0.38–1.66) compared with bilateral a-tDCSSham (Fig. 3).
Safety and side effects of a-tDCS
Participants' experiences and side effects were recorded at 0–5 min, 6–10 min, 11–15 min, and 16–20 min of stimulation. The means ± SEM of participant's reported side effects for all experimental sessions is summarized in Table 2. No side effects were found after a-tDCS other than light tingling sensations and itching under the electrodes during stimulation reported by some of the participants in all experimental conditions. Itching and tingling under the anode electrode were the most commonly reported side effects. Based on the result, the most severe tingling (mean value of 4.9 ± 0.15) at the beginning of dual-site stimulation and itching (mean value of 4.8 ± 0.45) at the beginning of M1 stimulation were recorded under the anode electrode. No adverse effects of a-tDCS such as burning sensations, headaches, or pain were detected during or after the single or concurrent bilateral dual-site stimulations.
Furthermore, Pearson's chi-square test was conducted to evaluate the success of blinding. The results showed no significant differences between the active and sham conditions (pM1 = 0.68, pCB = 0.75, pM1+CB = 0.69), demonstrating that participants could not differentiate between the active and sham stimulations. The majority of participants were properly blinded, and 75% of participants (excluding 'cannot say' responders) could not correctly guess the nature of the a-tDCS condition they had been received, which indicates that the blinding of the participants was successful in this study.
Discussion
This study compared the effects of a single session of concurrent bilateral a-tDCSM1+CB with bilateral a-tDCSM1 or bilateral a-tDCSCB on the CSE in twenty-one healthy young participants. The mechanisms behind the changes in CSE were also investigated using SICI, LICI, and ICF. The results indicate, all three stimulation conditions induced significant changes in CSE compared to its baseline (comparing Tpre and T0), while the bilateral a-tDCSM1 cause a significant decrease in the CSE level. In addition, the large effect sizes comparing the CSE after bilateral a-tDCSM1 with sham stimulation suggest a clinically meaningful reduction in the CSE level after bilateral a-tDCSM1 compared to the sham stimulation. In addition, the results show a large effect size when comparing bilateral a-tDCSCB and concurrent bilateral a-tDCSM1+CB to the sham stimulation, which indicates that these stimulation protocols are clinically meaningful for enhancing the CSE. Moreover, a lack of significant differences between the baseline assessments in different experimental conditions suggests that the length of the washing-out period to avoid the carry-over effect among the stimulation conditions was adequate. The results also showed that both single-site and dual-site applications of a-tDCS were well tolerated, and the blinding integrity was successfully achieved.
The effects of bilateral a-tDCSM1 on CSE
The results indicate a reduction in CSE level after bilateral a-tDCSM1. This study also demonstrated that the SICI was enhanced and the ICF reduced after bilateral a-tDCSM1, which explains this modulation. These changes suggesting the effects of bilateral a-tDCSM1 on CSE is inhibitory. Therefore, it may decrease the excitability of intracortical inhibitory interneurons and consequently increase the SICI level and decrease ICF level. Interestingly, these findings suggest that the 20 min of 2 mA a-tDCS of the M1 is shifting CSE from mechanisms associated with long term potentiation (LTP) plasticity, which was conventionally expected (Nitsche and Paulus 2000; Nitsche et al. 2005), to mechanisms associated with long term depression (LTD)-like plasticity. Although no study used bilateral a-tDCSM1 similar to this study to compare, the results of this study are in line with findings of other studies that used unilateral a- tDCSM1 conducted on healthy humans, which reported that the effects of a-tDCS are not linear and facilitatory, and depends on their parameters it can even decrease M1 CSE (Monte-Silva et al. 2009; Hassanzahraee et al. 2020).
The possible mechanisms behind these brain excitability alterations can be explained by the glutamatergic plasticity involving NMDA receptors (Liebetanz et al. 2002; Nitsche et al. 2003, 2004). Primarily, it has been shown that the activation of the NMDA receptors results in cellular calcium influx and thus affects synaptic plasticity. Based on the activation level of NMDA receptors, the leading effects on calcium influx and synaptic plasticity would be different. It has been provided that low calcium level results in LTD, high calcium increases induce LTP, and calcium overflow again results in LTD (Mosayebi Samani et al. 2019, 2020). Thus, it is speculated that 2 mA tDCS on M1 resulted in LTD-like plasticity due to calcium overflow. This calcium overflow may lead to counteracting potassium channel activation, limiting calcium influx (Yasuda et al. 2003; Misonou et al. 2004; Segal and Korkotian 2016) seems to convert effects. However, these explained mechanisms are speculative and should be explored and confirmed by future pharmacological studies.
The effects of bilateral a-tDCSCB on CSE
The results indicate an increase in the level of CSE after bilateral a-tDCSCB. Regarding cerebellar stimulation, this study showed the 2 mA, 20 min of a-tDCS is acting as an inhibitory technique on the CB, which inhibits the inhibitory effects of CB on M1 and facilitates the M1 increase in the level of CSE. In addition, this finding was supported by the rise in the level of ICF after bilateral a-tDCSCB compared to sham stimulation. These effects can be explained based on the physiology of the CB and M1 connections. Anatomically one of the main cerebellar efferent pathways to the M1 is called the cerebello-thalamo-cortical pathway (Holdefer et al. 2000; Grimaldi et al. 2014), arising from the cerebellar Purkinje cells to the M1 through the dentate nucleus and thalamus (Holdefer et al. 2000; Habas et al. 2009; Grimaldi et al. 2014; Tremblay et al. 2016; D'Angelo 2018). According to the inhibitory action of Purkinje cells, activation of Purkinje cells inhibits the dentate nucleus; the inhibited dentate cells send less excitatory stimuli to the ventrolateral thalamus and subsequently to the M1. Therefore, by inhibiting the CB, the Purkinje cells will be inhibited, and the inhibition of the dentate nucleus will be decreased. This means the dentate nucleus will send more excitatory stimuli to the thalamus and M1, and consequently, an increase in the level of CSE will be seen. Some recent reviews showed a lack of enough information surrounding the behavior of cerebellar a-tDCS on M1 excitability (Fernandez et al. 2018; Behrangrad et al. 2019). However, few studies evaluated the effects of the bilateral 2 mA, 20 min a-tDCS of the CB, and did not find any significant difference in the level of CSE (Galea et al. 2009; Bradnam et al. 2015; Craig and Doumas 2017; Summers et al. 2018; Ehrangrad et al. 2019).
One of the reasons can be the difference in electrode montage. According to the latest finding, it seems that placing the active electrode on the inion or a maximum of 1.5 cm below the inion increases the chance of stimulating the posterior and inferior parts of the CB (i.e., lobules VI–IIX) and hence significant effects after cerebellar a-tDCS (Behrangrad et al. 2019; Behrangrad 2021). The other possible reason seems to be the low number of participants included in these studies. The findings of this study shed light on the effects of cerebellar a-tDCS on the CSE. It is believed that interpreting the effects of the cerebellar a-tDCS on the M1 is not as simple as it seems, and more research is needed to be done to find the effects of cerebellar a-tDCS on the CSE and its behavior (Behrangrad et al. 2019).
The effects of concurrent bilateral a-tDCSM1+CB on CSE
According to the results, a significant increase, with a large effect size, was seen in CSE of the concurrent bilateral a-tDCSM1+CB compared to bilateral a-tDCSham. In addition, a significant increase in the CSE is seen after the concurrent bilateral a-tDCSM1+CB compared to baseline. This CSE increase can be explained by the significant increase found in the level of ICF of the concurrent bilateral a-tDCSM1+CB compared to bilateral a-tDCSham. It is speculated that the stimulation of M1 and CB reduces the GABAergic intracortical inhibition, which can be interpreted as a decrease in corticospinal neuron inhibition, causing an increased level of CSE. Furthermore, it is speculated that the concurrent bilateral a-tDCSM1+CB may shift the cortical excitability to LTP-like plasticity, which can be explained by increasing the activity of NMDA receptors and subsequent increase in calcium influx (Mosayebi Samani et al. 2019, 2020). This study is the first to investigate the effects of concurrent bilateral a-tDCSM1+CB, so further research is needed to support or disprove the results of this study.
Limitations of the study
The findings in this study should be interpreted considering its limitation. In this study, the effects of each stimulation condition were only assessed immediately after the interventions. This may limit our understanding of possible delayed plasticity changes. In addition, this study was carried out on young, healthy participants (between 18 and 40 years old); thus, the results may not be generalized to older adults or patients with pathological conditions that may not respond similarly to these techniques. In addition, because this study did not investigate gender as a variable, the results of this study are not gender-specific. In addition, although this study, as a proof-of-concept study, tried to investigate the underlying mechanisms behind the CSE changes of the stimulation conditions, this study could not completely disentangle whether CSE changes seen in dual-site stimulation is due to tDCS profound effect on the cerebellum or M1. Consequently, this could be an aim for future studies to have deeper understanding on the mechanisms behind the results found in this study.
Suggestions for future studies
Longer follow-ups are needed to evaluate the lasting effects of this stimulation technique. These data can be valuable for future studies investigating an optimal approach to improve the CSE of the M1. It is also essential to find out the effects of gender on the results of this stimulation technique in future research, to have more accurate and gender-specific results. It would be important to examine the effects of this a-tDCS technique on older adults and patients with different pathological conditions in future studies. In addition, future studies are necessary to investigate the behavioral outcome measures along with the neurophysiological changes.
Conclusion
The results of this study indicate that concurrent bilateral a-tDCSM1+CB and bilateral a-tDCSCB able to enhance M1 CSE and induce LTP-like plasticity. However, the results showed that the bilateral a-tDCSM1 stimulation might act as an inhibitory intervention rather than facilitatory, inducing LTD-like plasticity. In addition, this study showed that the effects of the a-tDCS for induction of increased CSE are not facilitatory all the time. Therefore, further investigations on the metaplastic mechanisms of this new approach are essential to produce efficient therapeutic neurorehabilitation protocols in healthy participants or patients who suffer from changes in the CSE and brain activity level in some neurological disorders affecting the M1 and CB circuits, such as stroke or multiple sclerosis.
As this technique (concurrent bilateral a-tDCSM1+CB) may potentially modulate brain function dramatically, investigating its effects is important for using it as a treatment in patients with brain connectivity disorders, such as cerebellar ataxia or Parkinson's.
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- a-tDCS:
-
Anodal transcranial direct current stimulation
- a-tDCSCB :
-
Bilateral a-tDCS of cerebellum
- a-tDCSM1 :
-
Bilateral a-tDCS of M1
- a-tDCSSham :
-
Bilateral sham a-tDCS
- a-tDCSM1+CB :
-
Concurrent bilateral a-tDCS of primary motor cortex and cerebellum
- CB:
-
Cerebellum
- CSE:
-
Corticospinal excitability
- CNS:
-
Central nervous system
- DLPFC:
-
Dorsolateral prefrontal cortex
- EMG:
-
Electromyography
- FDI:
-
First dorsal interosseous
- ICF:
-
Intracortical facilitation
- LICI:
-
Long intracortical inhibition
- M1:
-
Primary motor cortex
- MEP:
-
Motor evoked potential
- PEST:
-
Parameter estimation by sequential testing
- RMT:
-
Resting motor threshold
- SICI:
-
Short intracortical inhibition
- tDCS:
-
Transcranial direct current stimulation
- TMS:
-
Transcranial direct current stimulation
References
Allen GI, Tsukahara N (1974) Cerebrocerebellar communication systems. Physiol Rev 54(4):957–1006
Ammann C, Spampinato D, Márquez-Ruiz J (2016) Modulating motor learning through transcranial direct-current stimulation: an integrative view. Front Psychol 7:1981
Awiszus F (2003) TMS and threshold hunting. Suppl Clin Neurophysiol 56:13–23
Baharlouei HSE, Mehravar M, Manzari P, Shaterzadeh Yazdi MJ, Joghataei MT, Jaberzadeh S (2020) Comparison of transcranial direct current stimulation of the primary motor cortex and cerebellum on static balance in older adults. Iran Red Crescent Med J 22(3)
Behrangrad S (2021) The effect of a single-session of non-invasive brain stimulation on balance in healthy individuals: a systematic review and best evidence synthesis. Brain Connect
Behrangrad S, Zoghi M, Kidgell D, Jaberzadeh S (2019) Does cerebellar non-invasive brain stimulation affect corticospinal excitability in healthy individuals? A systematic review of literature and meta-analysis. Neurosci Lett 706:128–139
Bellebaum C, Daum I (2007) Cerebellar involvement in executive control. Cerebellum 6(3):184–192
Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J (2004) Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci 19(7):1950–1962
Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F (2007) Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci 25(2):123–129
Bostan AC, Dum RP, Strick PL (2013) Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 17(5):241–254
Bradnam LV, Graetz LJ, McDonnell MN, Ridding MC (2015) Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front Hum Neurosci 9:286
Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F (2011) A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 14(8):1133–1145
Carrillo F, Palomar FJ, Conde V, Diaz-Corrales FJ, Porcacchia P, Fernandez-Del-Olmo M, Koch G, Mir P (2013) Study of cerebello-thalamocortical pathway by transcranial magnetic stimulation in Parkinson’s disease. Brain Stimul 6(4):582–589
Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rosler KM, Triggs WJ, Ugawa Y, Ziemann U (2008) The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 119(3):504–532
Chen C, Fang Y, Wang X, Bao SC, Tang Z, Tong RK (2019) Excitation comparison between multi-site stimulation using network-based tDCS and focal stimulation using high-definition tDCS. Conf Proc IEEE Eng Med Biol Soc 2019:6884–6887
Cirillo G, Di Pino G, Capone F, Ranieri F, Florio L, Todisco V, Tedeschi G, Funke K, Di Lazzaro V (2017) Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul 10(1):1–18
Cohen J (1992) A power primer. Psychol Bull 112(1):155–159
Craig CE, Doumas M (2017) Anodal transcranial direct current stimulation shows minimal, measure-specific effects on dynamic postural control in young and older adults: a double blind, sham-controlled study. PLoS ONE 12(1):e0170331
D’Angelo E (2018) Physiology of the cerebellum. Handb Clin Neurol 154:85–108
Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt MA (2016) A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: influence of stimulation parameters. Brain Stimul 9(4):501–517
Devanne H, Cassim F, Ethier C, Brizzi L, Thevenon A, Capaday C (2006) The comparable size and overlapping nature of upper limb distal and proximal muscle representations in the human motor cortex. Eur J Neurosci 23(9):2467–2476
Dissanayaka T, Zoghi M, Farrell M, Egan GF, Jaberzadeh S (2017) Does transcranial electrical stimulation enhance corticospinal excitability of the motor cortex in healthy individuals? A systematic review and meta-analysis. Eur J Neurosci 46(4):1968–1990
Dissanayaka T, Zoghi M, Farrell M, Egan G, Jaberzadeh S (2018) Comparison of Rossini-Rothwell and adaptive threshold-hunting methods on the stability of TMS induced motor evoked potentials amplitudes. J Neurosci Res 96(11):1758–1765
Ehrangrad S, Zoghi M, Kidgell D, Jaberzadeh S (2019) Does cerebellar non-invasive brain stimulation affect corticospinal excitability in healthy individuals? A systematic review of literature and meta-analysis. Neurosci Lett 706:128–139
Ehsani F, Samaei A, Zoghi M, Hedayati R, Jaberzadeh S (2017) The effects of cerebellar transcranial direct current stimulation on static and dynamic postural stability in older individuals: a randomized double-blind sham-controlled study. Eur J Neurosci 46(12):2875–2884
Fernandez L, Major BP, Teo WP, Byrne LK, Enticott PG (2018) Assessing cerebellar brain inhibition (CBI) via transcranial magnetic stimulation (TMS): a systematic review. Neurosci Biobehav Rev 86:176–206
Ferrucci R, Cortese F, Priori A (2015) Cerebellar tDCS: how to do it. Cerebellum 14(1):27–30
Galea JM, Jayaram G, Ajagbe L, Celnik P (2009) Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 29(28):9115–9122
Gandiga PC, Hummel FC, Cohen LG (2006) Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol 117(4):845–850
George MS, Aston-Jones G (2010) Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology 35(1):301–316
Gilmore KL, Meyers JE (1983) Using surface electromyography in physiotherapy research. Aust J Physiother 29(1):3–9
Greenfield ML, Kuhn JE, Wojtys EM (1997) A statistics primer. Power analysis and sample size determination. Am J Sports Med 25(1):138–140
Grimaldi GAG, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, Galea JM, Groiss SJ, Hiraoka K, Kassavetis P, Lesage E, Manto M, Miall RC, Priori A, Sadnicka A, Ugawa Y, Ziemann U (2014) Non-invasive cerebellar stimulation—a consensus paper. Cerebellum 13:121–138
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD (2009) Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29(26):8586–8594
Hassanzahraee M, Nitsche MA, Zoghi M, Jaberzadeh S (2020) Determination of anodal tDCS duration threshold for reversal of corticospinal excitability: an investigation for induction of counter-regulatory mechanisms. Brain Stimul 13(3):832–839
Herbert R (2019) Research note: significance testing and hypothesis testing: meaningless, misleading and mostly unnecessary. J Physiother 65(3):178–181
Hickey GL, Grant SW, Dunning J, Siepe M (2018) Statistical primer: sample size and power calculations—why, when and how?". Eur J Cardiothorac Surg 54(1):4–9
Hill AT, Fitzgerald PB, Hoy KE (2016) Effects of anodal transcranial direct current stimulation on working memory: a systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stimul 9(2):197–208
Hill AT, Rogasch NC, Fitzgerald PB, Hoy KE (2018) Effects of single versus dual-site High-Definition transcranial direct current stimulation (HD-tDCS) on cortical reactivity and working memory performance in healthy subjects. Brain Stimul 11(5):1033–1043
Holdefer RN, Miller LE, Chen LL, Houk JC (2000) Functional connectivity between cerebellum and primary motor cortex in the awake monkey. J Neurophysiol 84:585–590
Horvath JC, Forte JD, Carter O (2015) Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS). Brain Stimul 8(3):535–550
Huang YZ, Lu MK, Antal A, Classen J, Nitsche M, Ziemann U, Ridding M, Hamada M, Ugawa Y, Jaberzadeh S, Suppa A, Paulus W, Rothwell J (2017) Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin Neurophysiol 128(11):2318–2329
Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U (2002) Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 545(1):153–167
Ishikawa T, Tomatsu S, Izawa J, Kakei S (2016) The cerebro-cerebellum: could it be loci of forward models?". Neurosci Res 104:72–79
Jung J, Bungert A, Bowtell R, Jackson SR (2020) Modulating brain networks with transcranial magnetic stimulation over the primary motor cortex: a concurrent TMS/fMRI study. Front Hum Neurosci 14:31
Kaminski E, Hoff M, Sehm B, Taubert M, Conde V, Steele CJ, Villringer A, Ragert P (2013) Effect of transcranial direct current stimulation (tDCS) during complex whole body motor skill learning. Neurosci Lett 552:76–80
Kaminski E, Steele CJ, Hoff M, Gundlach C, Rjosk V, Sehm B, Villringer A, Ragert P (2016) Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin Neurophysiol 127(6):2455–2462
Kaminski E, Hoff M, Rjosk V, Steele CJ, Gundlach C, Sehm B, Villringer A, Ragert P (2017) Anodal transcranial direct current stimulation does not facilitate dynamic balance task learning in healthy old adults. Front Hum Neurosci 11:16
Karabanov A, Ziemann U, Hamada M, George MS, Quartarone A, Classen J, Massimini M, Rothwell J, Siebner HR (2015) Consensus paper: probing homeostatic plasticity of human cortex with non-invasive transcranial brain stimulation. Brain Stimul 8(5):993–1006
Karok S, Witney AG (2013) Enhanced motor learning following task-concurrent dual transcranial direct current stimulation. PLoS ONE 8(12):e85693
Katsoulaki M, Kastrinis A, Tsekoura M (2017) The effects of anodal transcranial direct current stimulation on working memory. Adv Exp Med Biol 987:283–289
Koshy SM, Wiesman AI, Spooner RK, Embury C, Rezich MT, Heinrichs-Graham E, Wilson TW (2020) Multielectrode transcranial electrical stimulation of the left and right prefrontal cortices differentially impacts verbal working memory neural circuitry. Cereb Cortex 30(4):2389–2400
Krause B, Cohen Kadosh R (2014) Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front Syst Neurosci 8:25
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Labruna L, Jamil A, Fresnoza S, Batsikadze G, Kuo MF, Vanderschelden B, Ivry RB, Nitsche MA (2016) Efficacy of anodal transcranial direct current stimulation is related to sensitivity to transcranial magnetic stimulation. Brain Stimul 9(1):8–15
Li LM, Uehara K, Hanakawa T (2015) The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front Cell Neurosci 9:181
Liebetanz D, Nitsche MA, Tergau F, Paulus W (2002) Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125(Pt 10):2238–2247
Manor B, Zhou J, Harrison R, Lo OY, Travison TG, Hausdorff JM, Pascual-Leone A, Lipsitz L (2018) Transcranial direct current stimulation may improve cognitive-motor function in functionally limited older adults. Neurorehabil Neural Repair 32(9):788–798
Marquez J, Conley A, Karayanidis F, Lagopoulos J, Parsons M (2015) Anodal direct current stimulation in the healthy aged: effects determined by the hemisphere stimulated. Restor Neurol Neurosci 33(4):509–519
Martin DM, Moffa A, Nikolin S, Bennabi D, Brunoni AR, Flannery W, Haffen E, McClintock SM, Moreno ML, Padberg F, Palm U, Loo CK (2018) Cognitive effects of transcranial direct current stimulation treatment in patients with major depressive disorder: an individual patient data meta-analysis of randomised, sham-controlled trials. Neurosci Biobehav Rev 90:137–145
Medina J, Cason S (2017) No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex 94:131–141
Meyers LS, Gamst G, Guarino AJ (2006) Applied multivariate research: design and interpretation. SAGE Publications, Thousand Oaks
Monte-Silva K, Kuo MF, Thirugnanasambandam N, Liebetanz D, Paulus W, Nitsche MA (2009) Dose-dependent inverted U-shaped effect of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. J Neurosci 29(19):6124–6131
Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA (2013) Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 6(3):424–432
Morya E, Monte-Silva K, Bikson M, Esmaeilpour Z, Biazoli CE Jr, Fonseca A, Bocci T, Farzan F, Chatterjee R, Hausdorff JM, da Silva Machado DG, Brunoni AR, Mezger E, Moscaleski LA, Pegado R, Sato JR, Caetano MS, Sa KN, Tanaka C, Li LM, Baptista AF, Okano AH (2019) Beyond the target area: an integrative view of tDCS-induced motor cortex modulation in patients and athletes. J Neuroeng Rehabil 16(1):141
Mosayebi Samani M, Agboada D, Jamil A, Kuo MF, Nitsche MA (2019) Titrating the neuroplastic effects of cathodal transcranial direct current stimulation (tDCS) over the primary motor cortex. Cortex 119:350–361
Mosayebi Samani M, Agboada D, Kuo MF, Nitsche MA (2020) Probing the relevance of repeated cathodal transcranial direct current stimulation over the primary motor cortex for prolongation of after-effects. J Physiol 598(4):805–816
Nitsche MA (2011) Beyond the target area: remote effects of non-invasive brain stimulation in humans. J Physiol 589(Pt 13):3053–3054
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(Pt 3):633–639
Nitsche MA, Paulus W (2001) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57(10):1899–1901
Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W (2003) Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol 553(Pt 1):293–301
Nitsche MA, Liebetanz D, Schlitterlau A, Henschke U, Fricke K, Frommann K, Lang N, Henning S, Paulus W, Tergau F (2004) GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci 19(10):2720–2726
Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F (2005) Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol 568(Pt 1):291–303
Nomura T, Kirimoto H (2018) Anodal transcranial direct current stimulation over the supplementary motor area improves anticipatory postural adjustments in older adults. Front Hum Neurosci 12:317
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Pellegrini M, Zoghi M, Jaberzadeh S (2021a) Can genetic polymorphisms predict response variability to anodal transcranial direct current stimulation of the primary motor cortex?". Eur J Neurosci 53(5):1569–1591
Pellegrini M, Zoghi M, Jaberzadeh S (2021b) Genetic polymorphisms do not predict interindividual variability to cathodal transcranial direct current stimulation of the primary motor cortex. Brain Connect 11(1):56–72
Peters JC, Reithler J, Graaf TA, Schuhmann T, Goebel R, Sack AT (2020) Concurrent human TMS-EEG-fMRI enables monitoring of oscillatory brain state-dependent gating of cortico-subcortical network activity. Commun Biol 3(1):40
Pohjola H, Tolmunen T, Kotilainen T, Lehto SM (2017) Using transcranial direct current stimulation to enhance performance in balance tasks. Clin Neurophysiol 128:501–502
Poortvliet P, Hsieh B, Cresswell A, Au J, Meinzer M (2018) Cerebellar transcranial direct current stimulation improves adaptive postural control. Clin Neurophysiol 129(1):33–41
Rossini PM, Rossi S (1998) Clinical applications of motor evoked potentials. Electroencephalogr Clin Neurophysiol 106(3):180–194
Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS, Hallett M, Lefaucheur JP, Langguth B, Matsumoto H, Miniussi C, Nitsche MA, Pascual-Leone A, Paulus W, Rossi S, Rothwell JC, Siebner HR, Ugawa Y, Walsh V, Ziemann U (2015) Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 126(6):1071–1107
Saruco E, Di Rienzo F, Nunez-Nagy S, Rubio-Gonzalez MA, Jackson PL, Collet C, Saimpont A, Guillot A (2017) Anodal tDCS over the primary motor cortex improves motor imagery benefits on postural control: a pilot study. Sci Rep 7(1):480
Saruco E, Di Rienzo F, Nunez-Nagy S, Rubio-Gonzalez MA, Debarnot U, Collet C, Guillot A, Saimpont A (2018) Optimal combination of anodal transcranial direct current stimulations and motor imagery interventions. Neural Plast 2018:5351627
Schmidt S, Cichy RM, Kraft A, Brocke J, Irlbacher K, Brandt SA (2009) An initial transient-state and reliable measures of corticospinal excitability in TMS studies. Clin Neurophysiol 120(5):987–993
Shirota Y, Hamada M, Terao Y, Matsumoto H, Ohminami S, Furubayashi T, Nakatani-Enomoto S, Ugawa Y, Hanajima R (2010) Influence of short-interval intracortical inhibition on short-interval intracortical facilitation in human primary motor cortex. J Neurophysiol 104(3):1382–1391
Soekadar SR, Witkowski M, Cossio EG, Birbaumer N, Cohen LG (2014) Learned EEG-based brain self-regulation of motor-related oscillations during application of transcranial electric brain stimulation: feasibility and limitations. Front Behav Neurosci 8:93
Spampinato DA, Celnik PA, Rothwell JC (2020) Cerebellar-motor cortex connectivity: one or two different networks? J Neurosci 40:4230–4239
Steiner KM, Enders A, Thier W, Batsikadze G, Ludolph N, Ilg W, Timmann D (2016) Cerebellar tDCS does not improve learning in a complex whole body dynamic balance task in young healthy subjects. PLoS ONE 11(9):e0163598
Steiner KM, Thier W, Batsikadze G, Ludolph N, Ilg W, Timmann D (2020) Lack of effects of a single session of cerebellar transcranial direct current stimulation (tDCS) in a dynamic balance task. J Neurol 267(4):1206–1208
Summers RLS, Chen M, Hatch A, Kimberley TJ (2018) Cerebellar transcranial direct current stimulation modulates corticospinal excitability during motor training. Front Hum Neurosci 12:118
Tremblay S, Austin D, Hannah R, Rothwell JC (2016) Non-invasive brain stimulation as a tool to study cerebellar-M1 interactions in humans. Cerebellum Ataxias 3(19):1–23
Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M (1992) Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85(6):355–364
Vaseghi B, Zoghi M, Jaberzadeh S (2015) The effects of anodal-tDCS on corticospinal excitability enhancement and its after-effects: conventional vs. unihemispheric concurrent dual-site stimulation. Front Hum Neurosci 9:533
Verissimo IS, Barradas IM, Santos TT, Miranda PC, Ferreira HA (2016) Effects of prefrontal anodal transcranial direct current stimulation on working-memory and reaction time. Conf Proc IEEE Eng Med Biol Soc 2016:1790–1793
Vucic S, Ziemann U, Eisen A, Hallett M, Kiernan MC (2013) Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights. J Neurol Neurosurg Psychiatry 84(10):1161–1170
Wang B, Xiao S, Yu C, Zhou J, Fu W (2021) Effects of transcranial direct current stimulation combined with physical training on the excitability of the motor cortex, physical performance, and motor learning: a systematic review. Front Neurosci 15:648354
Wassermann EM (1998) Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 108(1):1–16
Wiltshire CEE, Watkins KE (2020) Failure of tDCS to modulate motor excitability and speech motor learning. Neuropsychologia 146:107568
Ziemann U, Rothwell JC, Ridding MC (1996) Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 496(Pt 3):873–881
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. No competing financial interests exist.
Author information
Authors and Affiliations
Contributions
Conceived and designed study: SB, MZ, DK, and SJ; performed data collection: SB; conducted the analysis: SB; interpreted the findings: SB, SJ; wrote the manuscript: SB; writing and editing of drafts: SB, MZ, DK, and SJ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Behrangrad, S., Zoghi, M., Kidgell, D. et al. The effects of concurrent bilateral anodal tDCS of primary motor cortex and cerebellum on corticospinal excitability: a randomized, double-blind sham-controlled study. Brain Struct Funct 227, 2395–2408 (2022). https://doi.org/10.1007/s00429-022-02533-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-022-02533-7