Abstract
Background
Externalizing and internalizing behaviors contribute to clinical impairment in children with neurodevelopmental disorders (NDDs). Although associations between externalizing or internalizing behaviors and cortico-amygdalar connectivity have been found in clinical and non-clinical pediatric samples, no previous study has examined whether similar shared associations are present across children with different NDDs.
Methods
Multi-modal neuroimaging and behavioral data from the Province of Ontario Neurodevelopmental Disorders (POND) Network were used. POND participants aged 6–18 years with a primary diagnosis of autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD) or obsessive–compulsive disorder (OCD), as well as typically developing children (TDC) with T1-weighted, resting-state fMRI or diffusion weighted imaging (DWI) and parent-report Child Behavioral Checklist (CBCL) data available, were analyzed (total n = 346). Associations between externalizing or internalizing behavior and cortico-amygdalar structural and functional connectivity indices were examined using linear regressions, controlling for age, gender, and image-modality specific covariates. Behavior-by-diagnosis interaction effects were also examined.
Results
No significant linear associations (or diagnosis-by-behavior interaction effects) were found between CBCL-measured externalizing or internalizing behaviors and any of the connectivity indices examined. Post-hoc bootstrapping analyses indicated stability and reliability of these null results.
Conclusions
The current study provides evidence towards an absence of a shared linear relationship between internalizing or externalizing behaviors and cortico-amygdalar connectivity properties across a transdiagnostic sample of children with different primary NDD diagnoses and TDC. Different methodological approaches, including incorporation of multi-dimensional behavioral data (e.g., task-based fMRI) or clustering approaches may be needed to clarify complex brain-behavior relationships relevant to externalizing/internalizing behaviors in heterogeneous clinical NDD populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), and pediatric obsessive–compulsive disorder (OCD) are neurodevelopmental disorders (NDDs) with high rates of clinical co-occurrence (Abramovitch et al. 2015; Jang et al. 2013; Lai et al. 2019; Lewin et al. 2011; Masi et al. 2006) in addition to significant overlap in clinical (Lawson et al. 2015; Mito et al. 2014), behavioral (Anholt et al. 2010; Havdahl et al. 2016), cognitive (Antshel et al. 2013; Van Der Meer et al. 2012), genetic (Lionel et al. 2014, 2011), and brain features (Ameis et al. 2016; Kern et al. 2015). This overlap has motivated recent research examining the shared and/or distinct biological and behavioral features across transdiagnostic samples (Ameis et al. 2016; Carlisi et al. 2017; Kushki et al. 2019). Externalizing (e.g., aggression, rule-breaking) and internalizing (e.g., withdrawal, anxiety, depression, somatic) behaviors manifest across children and youth to varying degrees (Bradley et al. 2004; Dwyer et al. 2006; Ghandour et al. 2019; Jacob et al. 2014). Children and youth with NDDs are more likely to exhibit clinically significant behaviors in either domain (Alvarenga et al. 2016; Bauminger et al. 2010; Jacob et al. 2014), contributing to increased functional impairment (e.g., at school and home) (Arim et al. 2015; Mazurek et al. 2013) and poorer response to interventions (Hill et al. 2014; Torp et al. 2015).
Internalizing and externalizing behaviors have been linked to alterations in various cortico-amygdalar networks, such as the parieto-amygdalar network (Karlsgodt et al. 2018; Chahal et al. 2020), default mode network (Umbach & Tottenham 2020; Sato et al. 2016), and the fronto-amygdalar network (Ameis et al. 2014; Vijayakumar et al. 2017). Frontal cortical regions in particular have been implicated in decision-making (Rushworth et al. 2011), behavioral regulation (Rushworth et al. 2011), and emotional regulation (Albaugh et al. 2016; Ducharme et al. 2011) which provide top-down modulation of amygdalar activity (Etkin et al. 2006; Hariri et al. 2003).This cortico-amygdalar network is connected through two main white matter tracts: the uncinate fasciculus (UF) and the cingulum bundle (CB) (Catani et al. 2013). In typically developing children (TDC), increased internalizing behavior has been associated with altered structural covariance between the prefrontal cortex and amygdala (Vijayakumar et al. 2017), decreased fractional anisotropy (FA) of the UF and CB (Albaugh et al. 2016; Mohamed Ali et al. 2019), and increased functional connectivity between the ventromedial prefrontal cortex and amygdala (Qin et al. 2014). Also in TDC, increased externalizing behavior has been associated with altered cortico-amygdalar structural covariance (Ameis et al. 2014), decreased FA of the UF (Andre et al. 2020), and altered functional connectivity between amygdala and frontal cortical regions (Aghajani et al. 2017, 2016; Saxbe et al. 2018). Broad cortico-amygdalar network alterations have also been found in studies of children with primary internalizing (e.g., major depressive disorder) or externalizing (e.g., oppositional defiant disorder) disorders (Castellanos-Ryan et al. 2014; Luking et al. 2011; Noordermeer et al. 2016; Paulesu et al. 2010; Stoycos et al. 2017) compared to TDC. Shared continuous associations between task-based fMRI and behavioral measures (parent report and in-scanner assessments) have also been found across children with different clinical diagnoses (i.e., disruptive behavior disorders, anxiety disorders, or ADHD) (Ibrahim et al. 2019; Stoddard et al. 2017). Taken together, these studies suggest that cortico-amygdalar connectivity properties may be associated with both externalizing and internalizing behaviors, which often co-occur (Korhonen et al. 2014; Reef et al. 2011), and may relate to these behaviors along a continuum cutting across TDC and different mental health diagnoses.
As of yet, we know of no study that has investigated whether cortico-amygdala network properties relate to internalizing or externalizing behaviors across children with different NDDs, which would suggest common neurobiological underpinnings contributing to these behaviors across diagnoses. The present study investigated linear associations between externalizing or internalizing behaviors and indices of cortico-amygdalar network connectivity (i.e., separately evaluated structural covariance, resting-state functional connectivity, and white matter connectivity) in a large sample, including TDC and children and youth with primary diagnoses of ASD, OCD, or ADHD. We hypothesized that greater externalizing or internalizing behaviors would be associated with reduced cortico-amygdalar structural and functional connectivity indices across our transdiagnostic sample.
Methods
Participants
Participants included in the current study participated in the Province of Ontario Neurodevelopmental Disorders (POND) Network; recruitment was carried out at different sites across the province of Ontario, Canada, including the Hospital for Sick Children (SickKids), Holland Bloorview Kids Rehabilitation Hospital, Lawson Health Research Institute, McMaster University and Queen’s University between June 2012 and January 2020. Children and youth were eligible to participate in POND if they had a primary clinical diagnosis of ASD, ADHD or OCD, sufficient English language comprehension to complete the behavioral assessments, and no contraindications for MRI (e.g., metal implants). The Parent Interview for Child Symptoms (Ickowicz et al. 2006) was used to confirm ADHD diagnosis, the Schedule for Affective Disorders-Children’s Version (Kiddie-SADS) and Children’s Yale-Brown Obsessive–Compulsive Scale (Scahill et al. 1997) for OCD, and the Autism Diagnostic Observation Schedule-2 (Lord et al. 2000) and the Autism Diagnostic Interview-Revised (Lord et al. 1994) for ASD. TDC participants were recruited through flyers posted at each recruitment site as well as through word-of-mouth. The exclusion criteria for TDC included: history of premature birth (< 35 weeks), presence of an NDD, first-degree relative with an NDD, psychiatric or neurologic diagnosis, confirmed via parental screening. Age-appropriate Wechsler scales were used to estimate full-scale IQ (Littell 1960). Participating institutions received approval for this study from their respective research ethics boards. Primary caregivers and participants provided either written informed consent or assent after a complete description of the study was provided. As of January 2020, MRI and behavioral data were available for 611 children and youth with ASD, ADHD, OCD, or TDC (n = 286 ASD; n = 159 ADHD; n = 68 OCD; n = 98 TDC) who completed MRI scanning at SickKids (Toronto, Canada). The present study analyzed data from a subset of these 611 POND participants who met all of the following criteria: (i) they had successfully completed a T1-weighted, resting-state, or single-shell DWI scan, (ii) were between the ages of 6 and 18 years at time of brain scan, and (iii) had Child Behavior Checklist (CBCL) data available that was collected within 12 months of their MRI scan (see Fig. 1).
Diagrams presenting the overall POND imaging samples which includes children with autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD) and typically developing children (TDC) scanned at the Hospital for Sick Children as of January 2020. Imaging data from T1-weighted (T1w), resting state fMRI (rsfMRI) and diffusion weighted imaging (DWI) sequences are presented. The reasons for exclusion presented for level 1: participants being outside the 6–18 age range at time of scan, a greater than 12 month time gap between scan and CBCL administration, and missing CBCL data; level 2: persistent processing errors at any point within the processing pipeline (e.g. errors in the fMRIprep pipeline); level 3: exclusion based on quality control (QC; details presented in the paper and supplement). The numbers for the final analysed sample for each imaging modality are presented. For the T1w and rs-fMRI samples, participants were scanned on a 3 T Siemens Tim Trio scanner prior to June 2016 when the scanner was upgraded to the PrismaFIT. For rs-fMRI acquisitions, participants scanned on the Tim Trio selected a movie to watch and participants scanned on the PrismaFIT viewed a naturalistic film (inscapes). The study includes only single-shell DWI acquisitions (n = 262) completed on the Tim Trio scanner
Measurement of externalizing and internalizing behaviors
Externalizing and internalizing behavioral scores were measured using the parent-report CBCL (ages 6–18), a standardized, well-established instrument (Achenbach and Ruffle 2000) that has been widely used for brain-behavior analyses in pediatric samples (Albaugh et al. 2016; Ameis et al. 2014; Ducharme et al. 2014, 2011; Ibrahim et al. 2019). The CBCL provides continuous measures of externalizing (calculated by combining rule-breaking and aggressive CBCL subscales) and internalizing behavior (calculated by combining withdrawn, anxious/depressed and somatic CBCL subscales), with a domain specific t-score (standardized by age and gender) > 70 indicating clinically significant symptoms.
MRI protocol
Participants were scanned on a 3 T Siemens Tim Trio at SickKids that was upgraded to the Siemens PrismaFIT in June 2016. All T1-weighted brain imaging consisted of a 5-min scan using an MPRAGE sequence with grappa parallelization (Tim Trio: (1 × 1x1)mm3, TR = 2,300 ms, TE = 2.96 ms, TI = 900 ms, Flip Angle = 9°, FOV = 224 × 224mm2, 240 Slices, GRAPPA = 2, 12-channel head coil; PrismaFIT: (0.8 × 0.8x0.8)mm3, TR = 1,870 ms, TE = 3.14 ms, TI = 945 ms, Flip Angle = 9°, FOV = 222 × 222mm2, 240 Slices, GRAPPA = 2, 20-channel head coil).
Resting state functional MRI (rs-fMRI) data consisted of a ~ 5-min scan (Tim Trio: (3.5 × 3.5x3.5)mm3, TR = 2340 ms, TE = 30 ms, Flip Angle = 70°, FOV = 256 × 240mm2, 120 volumes; PrismaFIT: (3 × 3x3)mm3, TR = 1500 ms, TE = 30 ms, Flip Angle = 70° FOV = 256 × 240mm2, 200 volumes). During rs-fMRI scanning, participants either viewed a movie of their choice, if the scan occurred pre-upgrade on Tim Trio, or a naturalistic movie paradigm (Vanderwal et al. 2015), if the scan occurred post-upgrade on PrismaFIT.
Single-shell DWI scans were acquired as 3 consecutive sequences with 19, 20 or 21 gradient directions (for a total of 60 directions) and 3 B0’s per acquisition sequence. Scan parameters were as follows: ((2 × 2x2)mm3, TR = 3800 ms, TE = 73 ms, Flip Angle = 90° FOV = 244 × 244mm2, 69 volumes, B0 = 1000). Multi-shell DWI data were acquired post scanner upgrade to PrismaFIT. Multi-shell data were not analyzed in the current study due to the challenges of harmonization across different DWI scan acquisition protocols and concerns regarding measurement variability given substantial differences in the pre-to-post hardware upgrade sequence design (Tax et al. 2019).
Image pre-processing
Prior to pre-processing, the acquired brain scans of participants who had multiple image acquisitions underwent quality assessment and the higher quality scan was pre-processed. Visual examination of the raw brain scan was used to assess the quality of T1-weighted and DWI acquisitions. Quality metric comparisons (e.g., mean framewise displacement [FD]) from the MRIQC pipeline (Esteban et al. 2017) was used to assess rs-fMRI acquisitions.
Structural MRI
T1-weighted brain images were pre-processed using the fMRIprep pipeline (Esteban et al. 2019) which runs FreeSurfer and performs intensity non-uniformity correction, skull stripping, calculates spatial normalization based on an MNI template, tissue segmentation and surface reconstruction. Images were also run through the MRIQC pipeline (Esteban et al. 2017) to extract quality metrics used in the quality control (QC) procedure. Left and right amygdala volumes from each participant were extracted using the amygdala region-of-interest (ROI) defined by the Desikan-Killiany Atlas (Desikan et al. 2006). The ciftify pipeline ((Dickie et al. 2019); https://github.com/edickie/ciftify) was used to transform the images from the FreeSurfer format to the Connectivity Informatics Technology Initiative (CIFTI) format. From there, the 40,962 vertices in each hemisphere were extracted based on FreeSurfer’s white and pial surfaces. This pipeline registered cortical surfaces to an average surface to establish correspondence between participants. Cortical thickness values at each vertex were smoothed with a Gaussian kernel of 12 mm full width half maximum (FWHM).
Resting state fMRI
The rs-fMRI acquisitions were pre-processed through fMRIPrep (Esteban et al. 2019). Within fMRIPrep, the data was slice timed and motion corrected. Distortion correction was performed using field maps; the functional image was co-registered to the corresponding T1-weighted image using FreeSurfer with boundary-based registration with 9 degrees of freedom. Nonlinear transformation to the MNI152 template was calculated via FSL’s FNIRT (based on the T1-weighted image) and applied to the functional data. These data were then transformed onto the cortical surface and converted to the CIFTI format (Dickie et al. 2019). The first three TRs were dropped, and voxel time series underwent mean-based intensity normalization, linear and quadratic detrending, temporal bandpass filtering (0.009–0.08 Hz), and confound regression for 6 head motion parameters, white matter signal, CSF signal and global signal plus their lags, their squares, and the squares of their lags (i.e. a 24HMP + 8 Phys + 4 GSR confounds) (Ciric et al. 2018). Global signal regression was employed as it has been shown to reduce sources of noise and reduce correlations between mean FD and functional connectivity (Parkes et al. 2018). Spatial smoothing was then performed on the cortical surface data (FWHM = 8 mm).
DWI
DWI scans from the three separate runs were concatenated. Diffusion data were denoised using random field theory and upsampled to a (1 × 1x1) mm3 voxel size using the MRtrix3 dwidenoise and mrresize commands, respectively (Veraart et al. 2016). Using fieldmaps, images were corrected for motion artefacts accounting for field inhomogeneities and eddy current induced artefacts using FSL's (Smith et al. 2004) eddy function (Andersson et al. 2016; Andersson and Sotiropoulos 2016). Deterministic tractography was used to delineate the UF and CB via the Slicer dMRI software (https://github.com/SlicerDMRI). The software registered the tracts for all participants using a dataset-specific atlas based on a representative subset (n = 21) from the current sample (selected based on age, gender and diagnosis) (Fedorov et al. 2012). Within the Slicer software, fiber clusters were manually appended to create the white matter tracts of interest (CB and UF). The atlas was registered to all participants’ DWI acquisitions and FA and mean diffusivity (MD) metrics were extracted.
Quality control (QC)
To reduce potential bias of image artefacts, a rigorous a priori QC procedure was applied for all imaging modalities (supplementary Sect. 1; Figure S1). T1-weighted images were assessed for motion artefacts using a visual QC approach (HTML visual outputs from the fMRIPrep pipeline) in addition to quantitative QC (MRIQC-derived quality metrics). For the rs-fMRI sequence, participants that did not complete the ~ 5-min scan were excluded based on prior research indicating this time duration is required for stable estimations of correlation strengths (Van Dijk et al. 2010). Quality of rs-fMRI acquisitions were assessed based on mean FD and excluded based on in-scanner motion at mean FD > 0.5 mm as implemented in prior studies (Satterthwaite et al. 2012; Choi et al. 2020) given that children have high levels of in-scanner head motion (Pardoe et al. 2016). DWI acquisitions were assessed for slice dropouts, poor V1 directions and residuals using an in-house standardized pipeline in addition to quantitative quality metrics. See Figure S1 and supplement for QC procedure details.
Statistical analysis
Brain-behavior relations
Each modality (T1w, rs-fMRI, and DWI) underwent a distinct statistical analytical pipeline. Separate linear regression models were fit to examine the presence of an association between externalizing or internalizing behavioral scores and cortico-amygdalar connectivity metrics from the three included modalities: structural covariance, seed based rs-fMRI, FA and MD of the UF and CB (see below and supplementary for details). Covariates for the primary regression models included age, gender, and scanner (if acquisitions from a modality included a scanner upgrade). Prior to fitting the brain-behavior regression models, linear regression models were fit between age, age-squared, and brain and behavior indices. The better fitting age term was included as a covariate in the main analyses (Table S2; supplementary Sect. 5.1). If the better fitting age term was quadratic, then linear and quadratic age terms were included in the model (see supplementary; Table S2, Figure S6). Across image modalities, if the primary regression models were significant, subsequent models were planned to sequentially fit the following covariates: (i) the alternate broad-band CBCL score (e.g., internalizing behavior as a covariate when externalizing behavior is the predictor variable) to account for shared variability (Zald and Lahey 2017), (ii) mean FD for functional connectivity models (Power et al. 2012; Satterthwaite et al. 2012) or an estimate of overall noise for white matter connectivity models (Anderson 2001) (see details in S3.2.1), and (iii) medication status (taking medication, not taking medication, unknown).
Cortico-amygdalar structural covariance
To be consistent with the approach used in prior studies examining the relationship between cortico-amygdalar connectivity and internalizing/externalizing behavior (Ameis et al. 2014; Ducharme et al. 2017; Albaugh et al. 2017; Vijayakumar et al. 2017), in the current study we assessed structural covariance using a vertex-wise approach. Using a partial regression, an interaction term (independent variable) between internalizing or externalizing behavior scores and left or right amygdala volume (e.g., externalizing behavior*left amygdala volume) was regressed onto each cortical vertex (with thickness at each vertex as the dependent variable) controlling for age, parent reported gender (boy/girl), intracranial volume (Buckner et al. 2004; Raz et al. 2004) and scanner (i.e., Tim Trio pre-upgrade or PrismaFIT post-upgrade). Analyses were carried out using FSL’s Permutation Analysis of Linear Models (PALM) package. Clusters of vertex-wise significance were determined using 2000 permutations with the threshold free cluster enhancement (TFCE) approach (Smith and Nichols 1996). Considering the high number of cortical vertices and consequent linear models, group results were thresholded at p < 0.05 FDR-corrected for the number of vertices in each hemisphere, and further corrected for separate runs of PALM for each hemisphere (critical level a = 0.025). Eight models were fit with cortical thickness at each vertex as the dependent variable to account for the different combinations between behavioral scores and amygdala volume and the behavior-by-diagnosis terms. Similar partial regression models were used for analyses examining rs-fMRI and DWI metrics as dependent variables. See below an example of one of the linear regression models examining associations between left cortico-amygdalar structural covariance and externalizing behavior.
Thickness at each cortical vertex = Intercept + β1(externalizing behavior CBCL score*left amygdala volume) + β2(externalizing behavior CBCL score) + β3(left amygdala volume) + β4(age) + β5(gender) + β6(intracranial volume) + β7(scanner) + ej.
Resting-state functional connectivity
Similar to prior studies examining the relationship between cortico-amygdalar connectivity and internalizing/externalizing behavior (Ibrahim et al. 2019; Stoddard et al. 2017), we assessed functional connectivity using a seed-based functional connectivity approach with the left and right amygdala as seed ROIs. Mean time series for each amygdala ROI were correlated with the time series of each cortical vertex using the ciftify_seed_corr function from the ciftify pipeline. Externalizing or internalizing behavior was regressed onto the functional connectivity between the amygdala ROI and each cortical vertex, controlling for age, gender, and scanner. PALM with TFCE was used to control for multiple comparisons across cortical vertices.
White matter connectivity
Using R (version 3.5.0), internalizing and externalizing behavioral scores were regressed onto left or right UF and CB for FA and MD metrics, controlling for age and gender. An FDR correction was applied to the primary analyses examining associations between behavior and left or right CB and UF diffusion metrics.
Interaction effects
To examine whether association patterns differed between diagnostic groups, behavior-by-diagnosis interaction terms were fit in separate models to examine whether brain-behavior relationships were influenced by diagnostic status.
Planned subsample analysis
Given the potential for considerable behavioral and brain change over time in a developing sample (Bos et al. 2018), a sensitivity analysis was conducted in a subset of participants whose brain scan was obtained within one month of completion of their CBCL data (see Figure S2 in supplemental materials for subsample details).
Post-hoc age-by-behavior interaction
Given that prior work has found age-specific relationships between externalizing/internalizing behaviors and cortico-amygdalar connectivity properties (Andre et al. 2019; Ducharme et al. 2014; Vijayakumar et al. 2017), age-by-behavior interaction terms were examined (see details in supplementary Sect. 8).
Post-hoc bootstrap resampling analysis
In light of recent calls for increased efforts to assess reliability of reported results (Button et al. 2013) due to the lack of replicability of neuroimaging research findings (Ioannidis, 2018; Simmons et al. 2011; He et al. 2020), we used bootstrap resampling to assess the stability and reliability for the brain-behavior models which address the main aims of the current study (i.e., models that examined the main effect of externalizing or internalizing behavior across cortico-amygdalar connectivity indices in the current sample). Using a case-resampling bootstrap (Monte Carlo) approach, 1000 iterations of each data (i.e., design) matrix were generated and used to perform repeated linear regressions for each generated sample. Each iteration of the data matrix randomly selected participants with replacement until the total sample size was reached (e.g., n = 346 for the main structural covariance models). Stability of the models were assessed using the bootstrap resampled standard errors of the regression coefficients (McIntosh and Lobaugh 2004; Efron and Tibshirani 1986). The reliability of assessed models was evaluated by examining the distributions (i.e., standard deviations) of the resampled model parameter estimates (i.e., regression coefficients, t-statistics, and effect sizes; Himberg et al. 2004). Stable and reliable models feature near-zero standard errors and low standard deviations of parameter estimates (McIntosh and Lobaugh 2004; Efron and Tibshirani 1986). For the structural covariance and functional connectivity models, bootstrap resampling was conducted in PALM and parameter estimates were calculated at each vertex. For the white matter connectivity models, each model was analyzed in RStudio and the standard errors of each model regression coefficients were calculated (see supplementary Sect. 7 for more details about the bootstrap resampling analysis). In addition to the bootstrap resampling analysis, a post-hoc power analysis was used to confirm that our study was adequately powered (see supplementary Sect. 8 for details and results of this analysis).
Results
Participant information
Characteristics of the analyzed sample are shown in Table 1. Following removal of participants outside the 6–18 year age range, those with a time window greater than 12 months between acquired scan and behavioral assessment date and those who failed QC, a total of 346 participants were included for structural covariance, 299 participants for resting-state functional connectivity and 157 participants for white matter connectivity analyses (see Fig. 1 for consort diagram). Across all image modalities, there were no significant differences in externalizing and internalizing behavior scores between the total POND sample scanned at SickKids by January 2020 (n = 611) and the subsample analyzed in the current study, nor were there any differences in age, gender, or diagnostic composition (see supplementary Sect. 4 for further details). A significant positive correlation between age and internalizing behaviors was found within each of the analyzed samples (T1w sample: r = 0.17, p < 0.001, rsfMRI sample: r = 0.15, p = 0.009, and DWI sample: r = 0.22, p = 0.005). A significant negative correlation between age and externalizing behaviors was found in the T1w sample (r = − 0.11, p = 0.044). There were no significant differences in age (F = 1.44, p = 0.24), internalizing (F = 0.03, p = 0.87) or externalizing (F = 0.41, p = 0.66) behaviors between participants included in T1w, rs-fMRI, and DWI samples. There were significant relationships between internalizing and externalizing scores across each of the imaging samples: T1w (r = 0.49, p < 0.001), rs-fMRI (r = 0.51, p < 0.001), and DWI samples (r = 0.53, p < 0.001). In the T1 and rs-fMRI samples, there was a significant difference in diagnostic composition across the scanner upgrade (X2 = 32.48, p < 0.001), consistent with the increased number of TDC participants that were scanned post upgrade.
Cortico-amygdalar structural covariance
No significant interaction effects between either internalizing or externalizing behavior score and left or right amygdala volume on vertex-wise cortical thickness were found. No significant effects were found when diagnostic status was included as an interaction term (i.e., diagnostic status-by-externalizing/internalizing behavior-by-left/right amygdala volume on cortical thickness). Figure 2 illustrates the unthresholded p-maps of the relationship between externalizing and internalizing behavior and left cortico-amygdalar structural covariance/functional connectivity.
Unthresholded spatial p-map of the relationship between externalizing/internalizing behaviors and cortico-amygdalar structural and functional connectivity. A Unthresholded spatial p-maps depicting the relationship between the interaction of externalizing and internalizing behavior and left amygdala volume on each cortical vertex. B Unthresholded spatial p-maps depicting the relationship between externalizing and internalizing behavior and functional connectivity between the left amygdala seed and each cortical vertex. A logp value of 1.6 is considered significant. As seen in the figure, none of the results reached this significance threshold
Functional and white matter connectivity
No significant associations were found between either externalizing or internalizing behavior and the time series correlations between left or right amygdala volume and each cortical vertex (Fig. 2), nor were there significant interaction effects found between externalizing or internalizing and diagnostic status on functional connectivity. Similarly, there were no significant associations found between either behavioral domain and FA or MD of the left or right UF or CB (Table 2, Fig. 3). No diagnosis-by-behavior interaction effects were found across the functional and white matter connectivity models.
Relationship between externalizing or internalizing behavior and fractional anisotropy and mean diffusivity (units: mm2/s) of the two white matter tracts of interest: the cingulum bundle and uncinate fasciculus. The depicted relationships are all non-significant. The black line is the regression line and the shaded gray area is the confidence interval. These figures include all data points, including potential outliers. Analyses were run with and without outlier removal; the results remained non-significant in either case. ADHD attention deficit hyperactivity disorder, ASD autism spectrum disorder, OCD obsessive compulsive disorder, CTRL healthy control/typically developing
Planned subsample analysis
Findings remained the same for the structural covariance and functional connectivity analyses among the subset of participants with a time-gap of one month or less between imaging acquisition and behavioral assessments. Among the white matter connectivity models, there was a significant association between MD of the right UF and externalizing behavior, however, this association no longer remained when participants with outlier MD values were removed (Figure S7). There was a main effect of externalizing behavior on the MD of the right CB, such that higher MD was associated with greater externalizing behavior (Figure S8) which did not survive the FDR significance threshold (F3,106 = 16.6, pModel < 0.001, tExternalizing = 2.19, pExternalizing = 0.03). There were no other significant associations found between FA or MD of the left or right UF or CB.
Post-hoc bootstrap resampling analysis
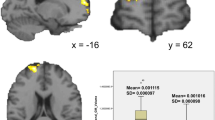
See Fig. 4 for structural covariance and functional connectivity bootstrap resampling analyses (and Figure S9 for other models) plotting the regression coefficients and their standard errors at each vertex (Panel A). Across all models, the regression coefficients based on 1000 generated bootstrapped resampled models were near zero (< 0.01; low signal) with respective standard errors near zero (< 0.001; low noise). Vertices with a t-statistic greater than an absolute value of 4 served as a proxy for a high signal vertex and are depicted in pink in Fig. 4. The figure illustrates the low standard errors of the regression coefficients (< 0.001) present for both high and low signal vertices, indicating that the results of the models examined in the current study are stable. As can be seen in Panels B-D, providing an example of bootstrap parameter estimates for models examining associations between externalizing behavior and cortico-left amygdalar structural and functional connectivity, the distribution (standard deviations) of the three mean model parameter estimates (i.e., regression coefficients, t-statistics and effect sizes, averaged across 1000 iterations) are centered around zero for both structural covariance and functional connectivity analyses. For the white matter connectivity models, the bootstrapped standard errors of the regression coefficients were also near zero and nearly all models featured bootstrapped confidence intervals which included zero (Table S5). See Figure S10 and Table S6 for bootstrap resampled results for the sensitivity analyses. The power analysis revealed that the current study was powered to detect effect sizes as small as d = 0.05–0.07 (see details in supplementary Sect. 8).
This figure depicts the bootstrap resampling results for the models examining associations between the externalizing behavior-left amygdala interaction term and whole brain structural covariance and functional connectivity. All other models examined feature similar results as those depicted here (see supplementary). In Panel A, the scatterplots illustrate associations between the mean regression coefficient (averaged across 1000 bootstrapped resamples) and the bootstrapped standard errors of the regression coefficients of each vertex for the structural covariance and functional connectivity models. Pink points depict the vertices with a higher signal (a t-statistic greater than 4). Blue points depict the vertices with low signal (t-statistic less than 4). The low standard errors found for both high and low signal vertices indicate stable results across resampling. Panel B depicts the histogram of the mean regression coefficients of each vertex across the 1000 bootstrapped resampled analyses (distribution–structural covariance: 2.56e-06 ± 1.37e-05; functional connectivity: 0.0002 ± 0.004). Panel C depicts the histogram of the mean t-statistic of each vertex across the 1000 bootstrapped resampled analyses (distribution–structural covariance: 0.357 ± 1.91; functional connectivity: 0.119 ± 1.99). Panel D depicts the histogram of the mean effect size of the model at each vertex across the 1000 bootstrapped resampled analyses (distribution—structural covariance: 8.45e-06 ± 4.66e-05; functional connectivity: 0.00045 ± 0.009). Note, all model parameter distributions (B-D) are centred around zero. The density y-axis in panels B-D figures is the number of points (i.e., vertices) that are in each histogram bin
Post-hoc age-by-behavior interaction effects
There were no age-by-externalizing or internalizing behavior interaction effects found on cortico-amygdalar structural covariance, functional connectivity, or white matter connectivity metrics (see supplementary Sect. 8, Figure S11, Table S7).
Discussion
Using a multi-modal imaging framework, the current study did not find continuous (dimensional) linear relationships between cortico-amygalar connectivity properties and externalizing or internalizing behaviors across a transdiagnostic sample including TDC and children with ASD, ADHD, or OCD. Further, our results do not suggest that the brain-behavior patterns examined differed by diagnostic group. The results of our post-hoc bootstrap resampling analyses indicate that the null results found in the present study are both stable and reliable.
Previous neuroimaging studies have found significant linear associations between internalizing or externalizing behavior and cortico-amygdalar connectivity metrics. Table S3 highlights studies which have examined this relationship with a focus on those that characterized externalizing and internalizing behaviors using the CBCL. Prior studies examining these relationships typically included small sample sizes (range: n = 21–291, with most studies including less than 100 participants). The majority of these studies conducted their analysis on typically developing samples, which feature limited variability of behavioral scores, when compared to a large transdiagnostic sample. Among TDC samples, lower rates of externalizing and internalizing behaviors have been shown to be associated with greater cortico-amygdalar connectivity across different modalities (e.g., increased structural covariance or functional connectivity between the amygdala and cortical regions). Importantly, studies that investigated this relationship in transdiagnostic samples found positive and negative relationships between externalizing/internalizing behaviors and functional connectivity (Chabernaud et al. 2012; Ibrahim et al. 2018), suggesting that brain-behavior relationship profiles may vary within heterogeneous samples. Among the prior studies included in Table S3, smaller effect sizes were found among studies with larger samples (Albaugh et al. 2016; Ameis et al. 2014; Vijayakumar et al. 2017). Further, many of these studies featured a narrower range of internalizing and externalizing behavioral scores across examined samples (e.g., current study range = 33–87 vs. T-score range ~ 30–70 in Ameis et al. 2014; Saxbe et al. 2018; Chabernaud et al. 2012; Karlsgodt et al. 2017; Qin et al. 2014). It is possible that the narrower behavioral heterogeneity compared to that included in the current study sample may have contributed to differences between the results reported here and findings of prior studies. Thus, the null results of the current study may not contradict prior findings as, to our knowledge, this is the only study examining the relationship between parent-reported externalizing/internalizing behaviors and multimodal cortico-amygdalar connectivity in a transdiagnostic NDD sample.
The current study featured a moderate-to-large sample size and was powered to detect very small effect sizes for each individual linear model (Cohen’s d ~ 0.04–0.07, supplementary Sect. 8), thus indicating that our null results were unlikely due to lack of power. Further, we examined the stability and reliability of our null results using bootstrap resampling. Using the bootstrap resampling analysis, instability of the model findings (which could be due to underpowered models and/or highly noisy data) can be detected through the standard errors of the model parameters. In the current study, our post-hoc bootstrap resampling analysis showed near-zero standard errors of regression coefficients for the main models (across both low and high signal vertices), indicating stability of the findings of the current study. The bootstrap resampling analysis indicated that the standard deviations for the model parameter estimates examined were also consistently centered around zero with low standard deviations, suggesting that the null models found in the current study are also reliable (McIntosh and Lobaugh, 2004; Efron and Tibshirani 1986; Himberg et al. 2004). Thus, the results of the power analysis and our bootstrap resampling provide further confidence that the current study was unlikely to be underpowered. Instead, the results of the power analysis in addition to the small effect sizes found across the bootstrap resampled models (d < 0.001; Fig. 4), could suggest that there is no meaningful linear relationship between externalizing or internalizing behavior, as measured, and cortico-amygdalar connectivity properties present across a heterogeneous transdiagnostic clinical sample of children with different NDDs.
Although the current study examined cortico-amygdalar connectivity across three imaging modalities, it is important to note that behavioral traits were assessed through a single broad-band parent-report measure. Delineating brain-behavior relations relevant to internalizing or externalizing behaviors in heterogeneous clinical samples may benefit from incorporating multi-modal measures of behavior (e.g., task-based fMRI using behavioral relevant tasks). Multi-modal measures of behavior may enhance measurement precision compared to the use of a parent-report behavioral measure alone. Two previous studies have examined brain-behavior relationships in transdiagnostic samples using both symptom measures and task-based fMRI. Ibrahim et al. found a negative association between cortico-amygdalar connectivity during an emotion perception task and externalizing behavior across children with ASD, with or without co-occurring disruptive behavior disorders (Ibrahim et al. 2019). Stoddard et al. found that amygdala-prefrontal cortical connectivity during viewing of intensely angry faces was associated with different behavioral profiles across a sample of children with ADHD, disruptive behavior disorders, anxiety disorders or TDC, whereby decreased connectivity was associated with high levels of anxiety and irritability, and lower connectivity was associated with high anxiety but low irritability (Stoddard et al. 2017).
A number of strengths and limitations of the current study require consideration. First, considering the growing concern of statistical practices which may contribute to false positive results or inflated effect sizes (Marek et al. 2020; Poldrack et al. 2017), we made use of non-parametric statistics (Eklund et al. 2016) (i.e. TFCE; (Smith and Nichols 1996)) to reduce risk of inflation for any potential associations found. Additionally, a standardized QC protocol was implemented across all imaging modalities to reduce the likelihood for findings to be driven by artefacts or motion (Backhausen et al. 2016; Pardoe et al. 2016). This resulted in an exclusion rate of 16.8–23.7% across imaging modalities based on image QC (that is, following initial exclusion of participants based on age, missing data and > 12-month time-gap between imaging and behavioral assessments), which is comparable to previous studies examining pediatric samples or using standardized QC approaches (Ameis et al. 2014; Ducharme et al. 2014; Xia et al. 2018) and in line with higher in-scanner motion in pediatric and clinical samples (Pardoe et al. 2016). While applying this rigorous QC approach is beneficial (particularly in a pediatric clinical sample), this limited our ability to leverage more of the total data available from POND. T1-weighted and rs-fMRI acquisitions for this sample were collected across a scanner upgrade, potentially introducing scanner-related confounds not captured by our statistical approaches. Further, while the parent-report behavioral measures of internalizing or externalizing behaviors used here have been used in prior brain-behavior studies (Albaugh et al. 2016; Ameis et al. 2014; Ducharme et al. 2014, 2011; Ibrahim et al. 2019), inclusion of additional measures (e.g., self-report behavioral or cognitive) may provide a more sensitive proxy of the behavioral domain of interest. Finally, given the increased variance present in our heterogeneous transdiagnostic NDD sample, which is likely present at both the behavioral and brain level (see Table 1, S3) (Dajani et al. 2016; Dickie et al. 2018; Fair et al. 2012), other analytic approaches including clustering methods and other data-driven algorithms (e.g., canonical correlation analysis or supervised machine learning (Lombardo et al. 2019; Feczko et al. 2019; Xia et al. 2019)) may be advantageous in future brain-behavior research than the more conventional univariate approaches (as in the linear models used here). Such analyses would need to establish whether newly identified clusters are robust and clinically meaningful. While employing such approaches is outside the scope of the aims of the current study, initial reports from cluster analyses applied to the POND sample indicate the potential for data-driven approaches to be useful in identifying subgroups of children with different NDD diagnoses (Jacobs et al. 2020; Kushki et al. 2019).
Conclusion
Producing consistent results that are generalizable and replicable has been challenging in clinical and cognitive neuroscience (Ioannidis, 2018; Simmons et al. 2011) as suggested by reports of non-replication (He et al. 2020) and inconsistent findings (Uddin et al. 2017) (Dajani et al. 2019; Masouleh et al. 2019). Null reports are necessary to refine methodological approaches which can inform future research. Contrary to our hypotheses, the stability and reliability of the null result found across three imaging modalities in the current study provides support for the absence of a dimensional linear association between externalizing or internalizing behavior and cortico-amygdalar connectivity across a heterogeneous group of children with different NDD diagnoses and TDCs. Future work exploring brain-behavior relations relevant to internalizing and externalizing domains in transdiagnostic samples may benefit from the use of additional clinical/cognitive/behavioral assessments (including multi-informant reports or relevant task-based fMRI), and data-driven analytical approaches to delineate subgroups with different brain-behavior profiles.
Data and code availability
See https://github.com/hajernakua/cortico-amygdalar2019 for the code used in our analyses (pre-processing and analysis scripts) and QC procedures. The POND Network has made a commitment to release the POND data. Data release is controlled and managed by the Ontario Brain Institute (OBI). The OBI POND data will be released via the Brain-CODE portal. For further details please see https://www.braincode.ca/.
References
Abramovitch A, Dar R, Mittelman A, Wilhelm S (2015) Comorbidity between attention deficit/hyperactivity disorder and obsessive-compulsive disorder across the lifespan: a systematic and critical review. Harv Rev Psychiatry 23:245–262. https://doi.org/10.1097/HRP.0000000000000050
Achenbach TM, Ruffle TM (2000) The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 21:265–271. https://doi.org/10.1542/pir.21-8-265
Aghajani M, Colins OF, Klapwijk ET, Veer IM, Andershed H, Popma A, van der Wee NJ, Vermeiren RRJM (2016) Dissociable relations between amygdala subregional networks and psychopathy trait dimensions in conduct-disordered juvenile offenders. Hum Brain Mapp 4033:4017–4033. https://doi.org/10.1002/hbm.23292
Aghajani M, Klapwijk ET, van der Wee NJ, Veer IM, Rombouts SARB, Boon AE, van Beelen P, Popma A, Vermeiren RRJM, Colins OF (2017) Disorganized amygdala networks in conduct-disordered juvenile offenders with callous-unemotional traits. Biol Psychiatry 82:283–293. https://doi.org/10.1016/j.biopsych.2016.05.017
Albaugh MD, Ducharme S, Watts R, Lewis JD (2016) Anxious/depressed symptoms are related to microstructural maturation of white matter in typically developing youths. Dev Psychopathol. https://doi.org/10.1017/S0954579416000444
Alvarenga PG, do Rosario MC, Cesar RC, Manfro GG, Moriyama TS, Bloch MH, Shavitt RG, Hoexter MQ, Coughlin CG, Leckman JF, Miguel EC (2016) Obsessive–compulsive symptoms are associated with psychiatric comorbidities, behavioral and clinical problems: a population-based study of Brazilian school children. Eur Child Adolesc Psychiatry 25:175–182. https://doi.org/10.1007/s00787-015-0723-3
Ameis SH, Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Lepage C, Zhao L, Khundrakpam B, Collins DL, Lerch JP, Wheeler A, Schachar R, Evans AC, Karama S (2014) Cortical thickness, cortico-amygdalar networks, and externalizing behaviors in healthy children. Biol Psychiatry 75:65–72. https://doi.org/10.1016/j.biopsych.2013.06.008
Ameis SH, Lerch JP, Taylor MJ, Lee W, Viviano JD, Pipitone J, Nazeri A, Croarkin PE, Voineskos AN, Lai MC, Crosbie J, Brian J, Soreni N, Schachar R, Szatmari P, Arnold PD, Anagnostou E (2016) A diffusion tensor imaging studyin children with ADHD, autism spectrum disorder, OCD, and matched controls: distinct and non-distinct white matter disruption and dimensional brain-behavior relationships. Am J Psychiatry 173:1213–1222. https://doi.org/10.1176/appi.ajp.2016.15111435
Anderson AW (2001) Theoretical analysis of the effects of noise on diffusion tensor imaging. Magn Reson Med 46:1174–1188. https://doi.org/10.1002/mrm.1315
Andersson JLR, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063–1078. https://doi.org/10.1016/j.neuroimage.2015.10.019
Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN (2016) Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 141:556–572. https://doi.org/10.1016/j.neuroimage.2016.06.058
Andre QR, Geeraert BL, Lebel C (2020) Brain structure and internalizing and externalizing behavior in typically developing children and adolescents. Brain Struct Funct. https://doi.org/10.1007/s00429-019-01973-y
Anholt GE, Cath DC, Van Oppen P, Eikelenboom M, Smit JH, Van Megen H, Van Balkom AJLM (2010) Autism and adhd symptoms in patients with ocd: are they associated with specific oc symptom dimensions or oc symptom severity. J Autism Dev Disord 40:580–589. https://doi.org/10.1007/s10803-009-0922-1
Antshel KM, Zhang-James Y, Faraone SV (2013) The comorbidity of ADHD and autism spectrum disorder. Expert Rev Neurother 13:1117–1128. https://doi.org/10.1586/14737175.2013.840417
Arim RG, Kohen DE, Garner RE, Lach LM, Brehaut JC, MacKenzie MJ, Rosenbaum PL (2015) Psychosocial functioning in children with neurodevelopmental disorders and externalizing behavior problems. Disabil Rehabil 37:345–354. https://doi.org/10.3109/09638288.2014.919361
Backhausen LL, Herting MM, Buse J, Roessner V, Smolka MN, Vetter NC (2016) Quality control of structural MRI images applied using FreeSurfer-a hands-on workflow to rate motion artifacts. Front Neurosci 10:1–10. https://doi.org/10.3389/fnins.2016.00558
Bauminger N, Solomon M, Rogers SJ (2010) Externalizing and internalizing behaviors in ASD. Autism Res 3:101–112. https://doi.org/10.1002/aur.131
Bos MGN, Wierenga LM, Blankenstein NE, Schreuders E, Tamnes CK, Crone EA (2018) Longitudinal structural brain development and externalizing behavior in adolescence. J Child Psychol Psychiatry Allied Discip 59:1061–1072. https://doi.org/10.1111/jcpp.12972
Bradley EA, Summers JA, Wood HL, Bryson SE (2004) Comparing rates of psychiatric and behavior disorders in adolescents and young adults with severe intellectual disability with and without autism. J Autism Dev Disord 34:151–161. https://doi.org/10.1023/B:JADD.0000022606.97580.19
Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ (2004) A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 23:724–738. https://doi.org/10.1016/j.neuroimage.2004.06.018
Button K, Ioannidis J, Mokrysz C et al (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14:365–376. https://doi.org/10.1038/nrn3475
Carlisi CO, Norman LJ, Lukito SS, Radua J, Mataix-Cols D, Rubia K (2017) Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol Psychiatry 82:83–102. https://doi.org/10.1016/j.biopsych.2016.10.006
Castellanos-Ryan N, Struve M, Whelan R, Banaschewski T, Barker GJ, Bokde ALW, Bromberg U, Büchel C, Flor H, Fauth-Bühler M, Frouin V, Gallinat J, Gowland P, Heinz A, Lawrence C, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Schumann G, Garavan H, Conrod PJ (2014) Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am J Psychiatry 171:1310–1319. https://doi.org/10.1176/appi.ajp.2014.13111499
Catani M, Dell’Acqua F, Thiebaut de Schotten M (2013) A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev 37:1724–1737. https://doi.org/10.1016/j.neubiorev.2013.07.001
Choi E, Vandewouw M, Taylor M, Arnold P, Brian J, Crosbie J, Kelley E, Lai M, Liu X, Schachar R, Lerch J, Anagnostou E (2020) Beyond diagnosis: cross-diagnostic features in canonical resting-state networks in children with neurodevelopmental disorders. Neuroimage Clin 28:102476. https://doi.org/10.1016/j.nicl.2020.102476
Ciric R, Rosen AFG, Erus G, Cieslak M, Adebimpe A, Cook PA, Bassett DS, Davatzikos C, Wolf DH, Satterthwaite TD (2018) Mitigating head motion artifact in functional connectivity MRI. Nat Protoc 13:2801–2826. https://doi.org/10.1038/s41596-018-0065-y
Dajani DR, Llabre MM, Nebel MB, Mostofsky SH, Uddin LQ (2016) Heterogeneity of executive functions among comorbid neurodevelopmental disorders. Sci Rep 6:1–10. https://doi.org/10.1038/srep36566
Dajani DR, Burrows CA, Odriozola P, Baez A, Nebel MB, Mostofsky SH, Uddin LQ (2019) Investigating functional brain network integrity using a traditional and novel categorical scheme for neurodevelopmental disorders. NeuroImage Clin 21:101678. https://doi.org/10.1016/j.nicl.2019.101678
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Dickie EW, Ameis SH, Shahab S, Calarco N, Smith DE, Miranda D, Viviano JD, Voineskos AN (2018) Personalized intrinsic network topography mapping and functional connectivity deficits in autism spectrum disorder. Biol Psychiatry 84:278–286. https://doi.org/10.1016/j.biopsych.2018.02.1174
Dickie EW, Anticevic A, Smith DE, Coalson TS, Manogaran M, Calarco N, Viviano JD, Glasser MF, Van Essen DC, Voineskos AN (2019) Ciftify: a framework for surface-based analysis of legacy MR acquisitions. Neuroimage 197:818–826. https://doi.org/10.1016/j.neuroimage.2019.04.078
Ducharme S, Hudziak JJ, Botteron KN, Ganjavi H, Lepage C, Collins DL, Albaugh MD, Evans AC, Karama S (2011) Right anterior cingulate cortical thickness and bilateral striatal volume correlate with child behavior checklist aggressive behavior scores in healthy children. Biol Psychiatry 70:283–290. https://doi.org/10.1016/j.biopsych.2011.03.015
Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Nguyen TV, Truong C, Evans AC, Karama S (2014) Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb Cortex 24:2941–2950. https://doi.org/10.1093/cercor/bht151
Dwyer SB, Nicholson JM, Battistutta D (2006) Parent and teacher identification of children at risk of developing internalizing or externalizing mental health problems: a comparison of screening methods. Prev Sci 7:343–357. https://doi.org/10.1007/s11121-006-0026-5
Efron B, Tibshirani R(1986) Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1(1):54–75. https://www.jstor.org/stable/2245500
Eklund A, Nichols TE, Knutsson H (2016) Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A 113:7900–7905. https://doi.org/10.1073/pnas.1602413113
Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ (2017) MRIQC: advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One 12:e0184661. https://doi.org/10.1371/journal.pone.0184661
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ (2019) fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16:111–116. https://doi.org/10.1038/s41592-018-0235-4
Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006) Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51:871–882. https://doi.org/10.1016/j.neuron.2006.07.029
Fair DA, Bathula D, Nikolas MA, Nigg JT (2012) Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A 109:6769–6774. https://doi.org/10.1073/pnas.1115365109
Feczko E, Miranda-Dominguez O, Marr M, Graham AM, Nigg JT, Fair DA (2019) The heterogeneity problem: approaches to identify psychiatric subtypes. Trends Cogn Sci 23:584–601. https://doi.org/10.1016/j.tics.2019.03.009
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30:1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Ghandour RM, Sherman LJ, Vladutiu CJ, Ali MM, Lynch SE, Bitsko RH, Blumberg SJ (2019) Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr 206:256-267.e3. https://doi.org/10.1016/j.jpeds.2018.09.021
Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR (2003) Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 53:494–501. https://doi.org/10.1016/S0006-3223(02)01786-9
Havdahl KA, von Tetzchner S, Huerta M, Lord C, Bishop SL (2016) Utility of the child behavior checklist as a screener for autism spectrum disorder. Autism Res 9:33–42. https://doi.org/10.1002/aur.1515
He Y, Byrge L, Kennedy DP (2020) Nonreplication of functional connectivity differences in autism spectrum disorder across multiple sites and denoising strategies. Hum Brain Mapp. https://doi.org/10.1002/hbm.24879
Hill AP, Zuckerman KE, Hagen AD, Kriz DJ, Duvall SW, Van Santen J, Nigg J, Fair D, Fombonne E (2014) Aggressive behavior problems in children with autism spectrum disorders: prevalence and correlates in a large clinical sample. Res Autism Spectr Disord 8:1121–1133. https://doi.org/10.1016/j.rasd.2014.05.006
Himberg J, Hyvarinen A, Esposito F (2004) Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 22:1214–1222. https://doi.org/10.1016/j.neuroimage.2004.03.027
Ibrahim K, Eilbott JA, Ventola P, He G, Pelphrey KA, McCarthy G, Sukhodolsky DG (2019) Reduced amygdala-prefrontal functional connectivity in children with autism spectrum disorder and co-occurring disruptive behavior. Biol Psychiatry Cogn Neurosci Neuroimaging 4:1031–1041. https://doi.org/10.1016/j.bpsc.2019.01.009
Ickowicz A, Schachar RJ, Sugarman R, Chen SX, Millette C, Cook L (2006) The parent interview for child symptoms. Can J Psychiatry 51:325–328. https://doi.org/10.1177/070674370605100508
Ioannidis JPA (2018) Why most published research findings are false. Get Good Res Integr Biomed Sci 2:2–8. https://doi.org/10.1371/journal.pmed.0020124
Jacob C, Gross-Lesch S, Jans T, Geissler J, Reif A, Dempfle A, Lesch KP (2014) Internalizing and externalizing behavior in adult ADHD. ADHD Atten Deficit Hyperact Disord 6:101–110. https://doi.org/10.1007/s12402-014-0128-z
Jacobs G, Voineskos A, Hawco C, Stefanik L, Forde N, Dickie E, Lai M-C, Szatmari P, Schachar R, Crosbie J, Arnold P, Goldenberg A, Erdman L, Lerch J, Anagnostou E, Ameis S (2020) Integration of brain and behavior measures for identification of data-driven groups cutting across children with ASD, ADHD, or OCD. Neuropsychopharmacol 46:647–653. https://doi.org/10.1038/s41386-020-00902-6
Jang J, Matson JL, Williams LW, Tureck K, Goldin RL, Cervantes PE (2013) Rates of comorbid symptoms in children with ASD, ADHD, and comorbid ASD and ADHD. Res Dev Disabil 34:2369–2378. https://doi.org/10.1016/j.ridd.2013.04.021
Kern JK, Geier DA, King PG, Sykes LK, Mehta JA, Geier MR (2015) Shared brain connectivity issues, symptoms, and comorbidities in autism spectrum disorder, attention deficit/hyperactivity disorder, and Tourette syndrome. Brain Connect 5:321–335. https://doi.org/10.1089/brain.2014.0324
Korhonen M, Luoma I, Salmelin RK, Helminen M, Kaltiala-Heino R, Tamminen T (2014) The trajectories of child’s internalizing and externalizing problems, social competence and adolescent self-reported problems in a Finnish normal population sample. Sch Psychol Int 35:561–579. https://doi.org/10.1177/0143034314525511
Kushki A, Anagnostou E, Hammill C, Duez P, Brian J, Iaboni A, Schachar R, Crosbie J, Arnold P, Lerch JP (2019) Examining overlap and homogeneity in ASD, ADHD, and OCD: a data-driven, diagnosis-agnostic approach. Transl Psychiatry. https://doi.org/10.1038/s41398-019-0631-2
Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, Szatmari P, Ameis SH (2019) Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry 6:819–829. https://doi.org/10.1016/S2215-0366(19)30289-5
Lawson RA, Papadakis AA, Higginson CI, Barnett JE, Wills MC, Strang JF, Wallace GL, Kenworthy L (2015) Everyday executive function impairments predict comorbid psychopathology in autism spectrum and attention deficit hyperactivity disorders. Neuropsychology 29:445–453. https://doi.org/10.1037/neu0000145
Lewin AB, Wood JJ, Gunderson S, Murphy TK, Storch EA (2011) Phenomenology of comorbid autism spectrum and obsessive-compulsive disorders among children. J Dev Phys Disabil 23:543–553. https://doi.org/10.1007/s10882-011-9247-z
Lionel AC, Crosbie J, Barbosa N, Goodale T, Thiruvahindrapuram B, Rickaby J, Gazzellone M, Carson AR, Howe JL, Wang Z, Wei J, Stewart AFR, Roberts R, McPherson R, Fiebig A, Franke A, Schreiber S, Zwaigenbaum L, Fernandez BA, Roberts W, Arnold PD, Szatmari P, Marshall CR, Schachar R, Scherere SW (2011) Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3002464
Lionel AC, Tammimies K, Vaags AK, Rosenfeld JA, Ahn JW, Merico D, Noor A, Runke CK, Pillalamarri VK, Carter MT, Gazzellone MJ, Thiruvahindrapuram B, Fagerberg C, Laulund LW, Pellecchia G, Lamoureux S, Deshpande C, Clayton-Smith J, White AC, Leather S, Trounce J, Bedford HM, Hatchwell E, Eis PS, Yuen RKC, Walker S, Uddin M, Geraghty MT, Nikkel SM, Tomiak EM, Fernandez BA, Soreni N, Crosbie J, Arnold PD, Schachar RJ, Roberts W, Paterson AD, So J, Szatmari P, Chrysler C, Woodbury-Smith M, Lowry RB, Zwaigenbaum L, Mandyam D, Wei J, MacDonald JR, Howe JL, Nalpathamkalam T, Wang Z, Tolson D, Cobb DS, Wilks TM, Sorensen MJ, Bader PI, An Y, Wu BL, Musumeci SA, Romano C, Postorivo D, Nardone AM, Monica MD, Scarano G, Zoccante L, Novara F, Zuffardi O, Ciccone R, Antona V, Carella M, Zelante L, Cavalli P, Poggiani C, Cavallari U, Argiropoulos B, Chernos J, Brasch-Andersen C, Speevak M, Fichera M, Ogilvie CM, Shen Y, Hodge JC, Talkowski ME, Stavropoulos DJ, Marshall CR, Scherer SW (2014) Disruption of the ASTN2/TRIM32 locus at 9q33.1 is a risk factor in males for autism spectrum disorders, ADHD and other neurodevelopmental phenotypes. Hum Mol Genet 23:2752–2768. https://doi.org/10.1093/hmg/ddt669
Littell WM (1960) The wechsler intelligence scale for children: review of a decade of research. Psychological Bulletin 57(2):132–156. https://doi.org/10.1037/h0044513
Lombardo M, Lai MC, Baron-Cohen S (2019) Big Data approaches to decomposing heterogeniety across the autism spectrum. Mol Psychiatry 24:1435–1450. https://doi.org/10.1038/s41380-018-0321-0
Lord C, Rutter M, Le Couteur A (1994) Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24(5):659–685. https://doi.org/10.1007/BF02172145
Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, Dilavore PC, Pickles A, Rutter M (2000) The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223. https://doi.org/10.1023/A:1005592401947
Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, Barch DM (2011) Functional connectivity of the amygdala in early-childhood-onset depression. J Am Acad Child Adolesc Psychiatry 50:1027-1041.e3. https://doi.org/10.1016/j.jaac.2011.07.019
Marek S, Tervo-Clemmens B, Calabro FJ et al (2022) Reproducible brain-wide association studies require thousands of individuals. Nature 603:654–660. https://doi.org/10.1038/s41586-022-04492-9
Masi G, Millepiedi S, Mucci M, Bertini N, Pfanner C, Arcangeli F (2006) Comorbidity of obsessive-compulsive disorder and attention-deficit/ hyperactivity disorder in referred children and adolescents. Compr Psychiatry 47:42–47. https://doi.org/10.1016/j.comppsych.2005.04.008
Masouleh SK, Eickhoff SB, Hoffstaedter F, Genon S (2019) Empirical examination of the replicability of associations between brain structure and psychological variables. Elife 8:1–25. https://doi.org/10.7554/eLife.43464
Mazurek MO, Kanne SM, Wodka EL (2013) Physical aggression in children and adolescents with autism spectrum disorders. Res Autism Spectr Disord 7:455–465. https://doi.org/10.1016/j.rasd.2012.11.004
McIntosh A, Lobaugh N (2004) Partial least square analysis of neuroimaging data: applications and advances. Neuroimage 23:S250–S263. https://doi.org/10.1016/j.neuroimage.2004.07.020
Mito H, Matsuura N, Mukai K, Yanagisawa Y, Nakajima A, Motoyama M, Arikawa A, Yamanishi K, Matsunaga H (2014) The impacts of elevated autism spectrum disorder traits on clinical and psychosocial features and long-term treatment outcome in adult patients with obsessive-compulsive disorder. Compr Psychiatry 55:1526–1533. https://doi.org/10.1016/j.comppsych.2014.05.005
Mohamed Ali O, Vandermeer MRJ, Sheikh HI, Joanisse MF, Hayden EP (2019) Girls’ internalizing symptoms and white matter tracts in Cortico-Limbic circuitry. NeuroImage Clin 21:101650. https://doi.org/10.1016/j.nicl.2018.101650
Noordermeer SDS, Luman M, Oosterlaan J (2016) A systematic review and meta-analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention-deficit hyperactivity disorder (ADHD) into account. Neuropsychol Rev 26:44–72. https://doi.org/10.1007/s11065-015-9315-8
Pardoe HR, Kucharsky Hiess R, Kuzniecky R (2016) Motion and morphometry in clinical and nonclinical populations. Neuroimage 135:177–185. https://doi.org/10.1016/j.neuroimage.2016.05.005
Parkes L, Fulcher B, Yücel M, Fornito A (2018) An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage 171:415–436. https://doi.org/10.1016/j.neuroimage.2017.12.073
Paulesu E, Sambugaro E, Torti T, Danelli L, Ferri F, Scialfa G, Sberna M, Ruggiero GM (2010) Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychol Med. https://doi.org/10.1017/S0033291709005649
Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafò MR, Nichols TE, Poline JB, Vul E, Yarkoni T (2017) Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 18:115–126. https://doi.org/10.1038/nrn.2016.167
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. https://doi.org/10.1016/j.neuroimage.2011.10.018
Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V (2014) Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry 75:892–900. https://doi.org/10.1016/j.biopsych.2013.10.006
Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD (2004) Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging 25:377–396. https://doi.org/10.1016/S0197-4580(03)00118-0
Reef J, Diamantopoulou S, Van Meurs I, Verhulst FC, Van Der Ende J (2011) Developmental trajectories of child to adolescent externalizing behavior and adult DSM-IV disorder: results of a 24-year longitudinal study. Soc Psychiatry Psychiatr Epidemiol 46:1233–1241. https://doi.org/10.1007/s00127-010-0297-9
Rushworth MFS, Noonan MAP, Boorman ED, Walton ME, Behrens TE (2011) Frontal cortex and reward-guided learning and decision-making. Neuron 70:1054–1069. https://doi.org/10.1016/j.neuron.2011.05.014
Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE (2012) Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage 60:623–632. https://doi.org/10.1016/j.neuroimage.2011.12.063
Saxbe D, Lyden H, Gimbel SI, Sachs M, Del Piero LB, Margolin G, Kaplan JT (2018) Longitudinal associations between family aggression, externalizing behavior, and the structure and function of the amygdala. J Res Adolesc 28:134–149. https://doi.org/10.1111/jora.12349
Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF (1997) Children’s Yale-brown obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 36:844–852. https://doi.org/10.1097/00004583-199706000-00023
Simmons JP, Nelson LD, Simonsohn U (2011) False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol Sci 22:1359–1366. https://doi.org/10.1177/0956797611417632
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44(1):83−98. https://doi.org/10.1016/j.neuroimage.2008.03.061
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:208–219. https://doi.org/10.1016/j.neuroimage.2004.07.051
Stoddard J, Tseng WL, Kim P, Chen G, Yi J, Donahue L, Brotman MA, Towbin KE, Pine DS, Leibenluft E (2017) Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiat 74:95–103. https://doi.org/10.1001/jamapsychiatry.2016.3282
Stoycos SA, Piero LD, Margolin G, Kaplan JT, Saxbe DE (2017) Neural correlates of inhibitory spillover in adolescence: associations with internalizing symptoms. Soc Cogn Affect Neurosci 12:1637–1646. https://doi.org/10.1093/scan/nsx098
Tax CM, Grussu F, Kaden E, Ning L, Rudrapatna U, John Evans C, St-Jean S, Leemans A, Koppers S, Merhof D, Ghosh A, Tanno R, Alexander DC, Zappalà S, Charron C, Kusmia S, Linden DE, Jones DK, Veraart J (2019) Cross-scanner and cross-protocol diffusion MRI data harmonisation: a benchmark database and evaluation of algorithms. Neuroimage 195:285–299. https://doi.org/10.1016/j.neuroimage.2019.01.077
Torp NC, Dahl K, Skarphedinsson G, Compton S, Thomsen PH, Weidle B, Hybel K, Valderhaug R, Melin K, Nissen JB, Ivarsson T (2015) Predictors associated with improved cognitive-behavioral therapy outcome in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 54:200-207.e1. https://doi.org/10.1016/j.jaac.2014.12.007
Uddin LQ, Dajani DR, Voorhies W, Bednarz H, Kana RK (2017) Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Transl Psychiatry 7:e1218. https://doi.org/10.1038/tp.2017.164
Van Der Meer JMJ, Oerlemans AM, Van Steijn DJ, Lappenschaar MGA, De Sonneville LMJ, Buitelaar JK, Rommelse NNJ (2012) Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J Am Acad Child Adolesc Psychiatry 51:1160-1172.e3. https://doi.org/10.1016/j.jaac.2012.08.024
Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010) Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103:297–321. https://doi.org/10.1152/jn.00783.2009
Vanderwal T, Kelly C, Eilbott J, Mayes LC, Castellanos FX (2015) Inscapes: a movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage 122:222–232. https://doi.org/10.1016/j.neuroimage.2015.07.069
Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E (2016) Denoising of diffusion MRI using random matrix theory. Neuroimage 142:394–406. https://doi.org/10.1016/j.neuroimage.2016.08.016
Vijayakumar N, Allen NB, Dennison M, Byrne ML, Simmons JG, Whittle S (2017) Cortico-amygdalar maturational coupling is associated with depressive symptom trajectories during adolescence. Neuroimage 156:403–411. https://doi.org/10.1016/j.neuroimage.2017.05.051
Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, Calkins ME, Cook PA, García de la Garza A, Vandekar SN, Cui Z, Moore TM, Roalf DR, Ruparel K, Wolf DH, Davatzikos C, Gur RC, Gur RE, Shinohara RT, Bassett DS, Satterthwaite TD (2018) Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun 9:1–14. https://doi.org/10.1038/s41467-018-05317-y
Zald D, Lahey B (2017) Implications of the hierarchical structure of psychopathology for psychiatric neuroimaging. Biol Psychiatry: CNNI 2:310–317. https://doi.org/10.1016/j.bpsc.2017.02.003
Acknowledgements
We thank the following individuals for research support and data collection: Tara Goodale, M.Sc., Reva Schachter, M.Sc., Mithula Sriskandarajah, B.Sc., Marlena Colasanto, M.Sc., Jennifer Gomez, M.A., and Laura Park, M.Sc, from The Hospital for Sick Children; Susan Day Fragiadakis, M.A., Naomi Peleg, M.Sc., and Leanne Ristic, B.A., from Holland Bloorview Kids Rehabilitation Hospital; Richa Mehta, B.A., Christina Sommerdyk, M.Sc., from the Lawson Health Research Institute; Carolyn Russell, B.Sc., Alessia Greco, M.A., Mike Chalupka, B.A., B.Sc., Christina Chrysler, B.A., Irene O’Connor, M.Ed. Psych., from McMaster Children’s Hospital, Melissa Hudson from Queen’s University. A special thank you to Alana Iaboni and Christopher Hammill for their extraordinary support in clarifying questions related to behavioral and imaging data, respectively, in the POND sample. An additional special thank you to Dr. Anthony Randal McIntosh who provided assistance with constructing and interpreting the bootstrap resampling analysis.
Funding
This research was conducted with the support of the Ontario Brain Institute (POND, PIs: Anagnostou/Lerch), an independent non-profit corporation, funded partially by the Ontario government. The opinions, results and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. HN has received funding from the CAMH Discovery Fund and currently receives funding from the Ontario Graduate Scholarship. GRJ received funding from the Ontario Graduate Scholarship and Ontario Student Opportunity Trust Fund. ANV currently receives funding from the National Institute of Mental Health (1/3R01MH102324 and 1/5R01MH114970), Canadian Institutes of Health Research, Canada Foundation for Innovation, CAMH Foundation, and University of Toronto. NJF received funding from the Centre for Addiction and Mental Health Discovery Fund Postdoctoral Grant. M-CL receives funding from the Ontario Brain Institute via the POND Network, Canadian Institutes of Health Research, the Academic Scholars Award from the Department of Psychiatry, University of Toronto, and CAMH Foundation. PS has received royalties from Guilford Press. RS has consulted to Highland Therapeutics, Eli Lilly and Co., and Purdue Pharma. He has commercial interest in a cognitive rehabilitation software company, “eHave”. PDA receives funding from the Alberta Innovates Translational Health Chair in Child and Youth Mental Health and holds a patent for ‘SLCIAI Marker for Anxiety Disorder’ granted May 6, 2008. EA receives funding from the Canadian Institutes of Health Research, National Institutes of Health, Ontario Brain Institute, Brain Canada, Azrieli foundation, Autism Speaks, Health Resources and Services Administration. She has served as a consultant to Roche and Quadrant, has received grant funding from Roche, holds a patent for the device, “Anxiety Meter”, has received editorial honoria from Wiley and royalties from APPI and Springer. SHA currently receives funding from the National Institute of Mental Health (R01MH114879), Canadian Institutes of Health Research, the Academic Scholars Award from the Department of Psychiatry, University of Toronto, Autism Speaks Canada and the CAMH Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Other authors report no related funding support, financial or potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakua, H., Hawco, C., Forde, N.J. et al. Cortico-amygdalar connectivity and externalizing/internalizing behavior in children with neurodevelopmental disorders. Brain Struct Funct 227, 1963–1979 (2022). https://doi.org/10.1007/s00429-022-02483-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-022-02483-0