Abstract
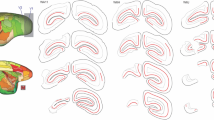
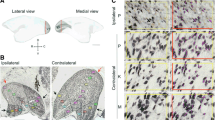
Lesions in the primary visual cortex (V1) cause extensive retrograde degeneration in the lateral geniculate nucleus, but it remains unclear whether they also trigger any neuronal loss in other subcortical visual centers. The inferior (IPul) and lateral (LPul) pulvinar nuclei have been regarded as part of the pathways that convey visual information to both V1 and extrastriate cortex. Here, we apply stereological analysis techniques to NeuN-stained sections of marmoset brain, in order to investigate whether the volume of these nuclei, and the number of neurons they comprise, change following unilateral long-term V1 lesions. For comparison, the medial pulvinar nucleus (MPul), which has no connections with V1, was also studied. Compared to control animals, animals with lesions incurred either 6 weeks after birth or in adulthood showed significant LPul volume loss following long (> 11 months) survival times. However, no obvious areas of neuronal degeneration were observed. In addition, estimates of neuronal density in lesioned hemispheres were similar to those in the non-lesioned hemispheres of same animals. Our results support the view that, in marked contrast with the geniculocortical projection, the pulvinar pathway is largely spared from the most severe long-term effects of V1 lesions, whether incurred in early postnatal or adult life. This difference can be linked to the more divergent pattern of pulvinar connectivity to the visual cortex, including strong reciprocal connections with extrastriate areas. The results also caution against interpretation of volume loss in brain structures as a marker for neuronal degeneration.









Similar content being viewed by others
References
Adams MM, Hof PR, Gattass R, Webster MJ, Ungerleider LG (2000) Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J Comp Neurol 419:377–393
Asami T, Yoshida H, Takaishi M, Nakamura R, Yoshimi A, Whitford TJ, Hirayasu Y (2018) Thalamic shape and volume abnormalities in female patients with panic disorder. PLoS One 13:e0208152
Atapour N, Worthy KH, Lui LL, Yu HH, Rosa MGP (2017) Neuronal degeneration in the dorsal lateral geniculate nucleus following lesions of primary visual cortex: comparison of young adult and geriatric marmoset monkeys. Brain Struct Funct 222:3283–3293
Atapour N, Majka P, Wolkowicz IH, Malamanova D, Worthy KH, Rosa MGP (2019) Neuronal distribution across the cerebral cortex of the marmoset monkey (Callithrix jacchus). Cereb Cortex 29:3836–3863
Atapour N, Worthy KH, Rosa MGP (2021) Neurochemical changes in the primate lateral geniculate nucleus following lesions of striate cortex in infancy and adulthood: implications for residual vision and blindsight. Brain Struct Funct. https://doi.org/10.1007/s00429-021-02257-0
Baldwin MKL, Balaram P, Kaas JH (2017) The evolution and functions of nuclei of the visual pulvinar in primates. J Comp Neurol 525:3207–3226
Beck PD, Kaas JH (1998) Thalamic connections of the dorsomedial visual area in primates. J Comp Neurol 396(3):381–398
Benevento L, Rezak M (1976) The cortical projections of the inferior pulvinar and adjacent lateral pulvinar in the rhesus monkey (Macaca mulatta): an autoradiographic study. Brain Res 108:1–24
Berman RA, Wurtz RH (2010) Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci 30:6342–6354
Berman RA, Wurtz RH (2011) Signals conveyed in the pulvinar pathway from superior colliculus to cortical area MT. J Neurosci 31:373–384
Bourne JA, Morrone MC (2017) Plasticity of visual pathways and function in the developing brain: is the pulvinar a crucial player? Front Syst Neurosci 11:3
Bourne JA, Rosa MGP (2006) Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: early maturation of the middle temporal area (MT). Cereb Cortex 16:405–414
Bourne JA, Warner CE, Rosa MGP (2005) Topographic and laminar maturation of striate cortex in early postnatal marmoset monkeys, as revealed by neurofilament immunohistochemistry. Cereb Cortex 15:740–748
Bridge H, Leopold DA, Bourne JA (2016) adaptive pulvinar circuitry supports visual cognition. Trends Cogn Sci 20:146–157
Bridge H, Bell AH, Ainsworth M, Sallet J, Premereur E, Ahmed B, Mitchell AS, Schüffelgen U, Buckley M, Tendler BC, Miller KL (2019) Preserved extrastriate visual network in a monkey with substantial, naturally occurring damage to primary visual cortex. Elife 8:e42325
Bullier J, Kennedy H (1983) Projection of the lateral geniculate nucleus onto cortical area V2 in the macaque monkey. Exp Brain Res 53:168–172
Burman KJ, Bakola S, Richardson KE, Yu HH, Reser DH, Rosa MGP (2015) Cortical and thalamic projections to cytoarchitectural areas 6Va and 8C of the marmoset monkey: connectionally distinct subdivisions of the lateral premotor cortex. J Comp Neurol 523:1222–1247
Byne W, Fernandes J, Haroutunian V, Huacon D, Kidkardnee S, Kim J, Tatusov A, Thakur U, Yiannoulos G (2007) Reduction of right medial pulvinar volume and neuron number in schizophrenia. Schizophr Res 90:71–75
Chalfin BP, Cheung DT, Muniz JA, de Lima Silveira LC, Finlay BL (2007) Scaling of neuron number and volume of the pulvinar complex in New World primates: comparisons with humans, other primates, and mammals. J Comp Neurol 504:265–274
Chaplin TA, Yu HH, Rosa MGP (2013) Representation of the visual field in the primary visual area of the marmoset monkey: magnification factors, point-image size, and proportionality to retinal ganglion cell density. J Comp Neurol 521:1001–1019
Chen D, Huang Y, Shi Z, Li J, Zhang Y, Wang K, Smith AD, Gong Y, Gao Y (2020) Demyelinating processes in aging and stroke in the central nervous system and the prospect of treatment strategy. CNS Neurosci Ther 26:1219–1229
Cowey A, Alexander I, Stoerig P (2011) Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain 134:2149–2157
Cusick CG, Gould HJ III (1990) Connections between area 3b of the somatosensory cortex and subdivisions of the ventroposterior nuclear complex and the anterior pulvinar nucleus in squirrel monkeys. J Comp Neurol 292:83–102
Cusick CG, Scripter JL, Darensbourg JG, Weber JT (1993) Chemoarchitectonic subdivisions of the visual pulvinar in monkeys and their connectional relations with the middle temporal and rostral dorsolateral visual areas, MT and DLr. J Comp Neurol 336:1–30
Dick A, Kaske A, Creutzfeldt OD (1991) Topographical and topological organization of the thalamocortical projection to the striate and prestriate cortex in the marmoset (Callithrix jacchus). Exp Brain Res 84:233–253
Dineen JT, Hendrickson AE (1981) Age correlated differences in the amount of retinal degeneration after striate cortex lesions in monkeys. Invest Ophthalmol vis Sci 21:749–752
Fox DM, Goodale MA, Bourne JA (2020) The age-dependent neural substrates of blindsight. Trends Neurosci 43:242–252
Gallyas F (1979) Silver staining of myelin by means of physical development. Neurol Res 1:203–209
Gao X, Deng P, Xu ZC, Chen J (2011) Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS One 6(9):e24566
Grieve KL, Acuña C, Cudeiro J (2000) The primate pulvinar nuclei: vision and action. Trends Neurosci 23:35–39
Gundersen HJ, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147:229–263
Gutierrez C, Cola MG, Seltzer B, Cusick C (2000) Neurochemical and connectional organisation of the dorsal pulvinar complex in monkeys. J Comp Neurol 419:61–86
Hagan MA, Rosa MGP, Lui LL (2017) Neural plasticity following lesions of the primate occipital lobe: the marmoset as an animal model for studies of blindsight. Dev Neurobiol 77:314–327
Hagan MA, Chaplin TA, Huxlin KR, Rosa MGP, Lui LL (2019) Altered sensitivity to motion of area MT neurons following long-term V1 lesions. Cereb Cortex 30:451–464
Harting JK, Huerta MF, Frankfurter AJ, Strominger NL, Royce GJ (1980) Ascending pathways from the monkey superior colliculus: an autoradiographic analysis. J Comp Neurol 192:853–882
Hendrickson A, Warner CE, Possin D, Huang J, Kwan WC, Bourne JA (2015) Retrograde transneuronal degeneration in the retina and lateral geniculate nucleus of the V1-lesioned marmoset monkey. Brain Struct Funct 220:351–360
Hill CS, Coleman MP, Menon DK (2016) Traumatic axonal injury: mechanisms and translational opportunities. Trends Neurosci 39:311–324
Homman-Ludiye J, Mundinano IC, Kwan WC, Bourne JA (2020) Extensive connectivity between the medial pulvinar and the cortex revealed in the marmoset monkey. Cereb Cortex 30:1797–1812
Huo BX, Zeater N, Lin MK, Takahashi YS, Hanada M, Nagashima J, Lee BC, Hata J, Zaheer A, Grünert U, Miller MI, Rosa MGP, Okano H, Martin PR, Mitra PP (2019) Relation of koniocellular layers of dorsal lateral geniculate to inferior pulvinar nuclei in common marmosets. Eur J Neurosci 50:4004–4017
Jones EG (2001) The thalamic matrix and thalamocortical synchrony. Trends Neurosci 24(10):595–601
Jones EG (2007) The thalamus, 2nd edn. Cambridge University Press, Cambridge
Kaas JH, Baldwin MKL (2020) The evolution of the pulvinar complex in primates and its role in the dorsal and ventral streams of cortical processing. Vision (basel) 4:3
Kaas JH, Lyon DC (2007) Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev 55:285–296
Kaske A, Dick A, Creutzfeldt OD (1991) The local domain for divergence of subcortical afferents to the striate and extrastriate visual cortex in the common marmoset (Callithrix jacchus): a multiple labelling study. Exp Brain Res 84:254–265
Kemether EM, Buchsbaum MS, Byne W, Hazlett EA, Haznedar M, Brickman AM, Platholi J, Bloom R (2003) Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with Schizophrenia. Arch Gen Psychiatry 60:983–991
Kennedy H, Bullier J (1985) A double-labeling investigation of the afferent connectivity to cortical areas V1 and V2 of the macaque monkey. J Neurosci 5:2815–2830
Krishnamurthy LC, Champion GN, McGregor KM, Krishnamurthy V, Turabi A, Roberts SR, Nocera JR, Borich MR, Rodriguez AD, Belagaje SR, Harrington RM, Harris-Love ML, Harnish SM, Drucker JH, Benjamin M, Meadows ML, Seeds L, Zlatar ZZ, Sudhyadhom A, Butler AJ, Garcia A, Patten C, Trinastic J, Kautz SA, Gregory C, Crosson BA (2020) The effect of time since stroke, gender, age, and lesion size on thalamus volume in chronic stroke: a pilot study. Sci Rep 10:20488
Lysakowski A, Standage GP, Benevento LA (1988) An investigation of collateral projections of the dorsal lateral geniculate nucleus and other subcortical structures to cortical areas V1 and V4 in the macaque monkey: a double label retrograde tracer study. Exp Brain Res 69:651–661
Magon S, May A, Stankewitz A, Goadsby PJ, Tso AR, Ashina M, Amin FM, Seifert CL, Chakravarty MM, Müller J, Sprenger T (2015) Morphological abnormalities of thalamic subnuclei in migraine: a multicenter MRI study at 3 Tesla. J Neurosci 35:13800–13806
Mahajan KR, Nakamura K, Cohen JA, Trapp BD, Ontaneda D (2020) Intrinsic and extrinsic mechanisms of thalamic pathology in multiple sclerosis. Ann Neurol 88:81–92
Majka P, Bednarek S, Chan JM, Jermakow N, Liu C, Saworska G, Worthy KH, Silva AC, Wójcik DK, Rosa MGP (2021) Histology-based average template of the marmoset cortex with probabilistic localization of cytoarchitectural areas. Neuroimage 226:117625
Marion CM, McDaniel DP, Armstrong RC (2019) Sarm1 deletion reduces axon damage, demyelination, and white matter atrophy after experimental traumatic brain injury. Exp Neurol 321:113040
Meier PG, Maeder P, Kardon RH, Borruat FX (2015) Homonymous ganglion cell layer thinning after isolated occipital lesion: macular OCT demonstrates transsynaptic retrograde retinal degeneration. J Neuroophthalmol 35:112–116
Melnick MD, Tadin D, Huxlin KR (2016) Relearning to see in cortical blindness. Neuroscientist 22:199–212
Minagar A, Barnett MH, Benedict RH, Pelletier D, Pirko I, Sahraian MA, Frohman E, Zivadinov R (2013) The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology 80:210–219
Missler M, Eins S, Merker HJ, Rothe H, Wolff JR (1993a) Pre- and postnatal development of the primary visual cortex of the common marmoset. I. A changing space for synaptogenesis. J Comp Neurol 333:41–52
Missler M, Wolff A, Merker HJ, Wolff JR (1993b) Pre- and postnatal development of the primary visual cortex of the common marmoset. II. Formation, remodelling, and elimination of synapses as overlapping processes. J Comp Neurol 333:53–67
Mundinano IC, Kwan WC, Bourne JA (2019) Retinotopic specializations of cortical and thalamic inputs to area MT. Proc Natl Acad Sci USA 116:23326–23331
Ogren M, Hendrickson A (1976) Pathways between striate cortex and subcortical regions in Macaca mulatta and Saimiri sciureus: evidence for a reciprocal pulvinar connection. Exp Neurol 53(3):780–800
Pareto D, Garcia-Vidal A, Alberich M, Auger C, Montalban X, Tintoré M, Sastre-Garriga J, Rovira À (2020) Ratio of T1-weighted to T2-weighted signal intensity as a measure of tissue integrity: comparison with magnetization transfer ratio in patients with multiple sclerosis. Am J Neuroradiol 41:461–463
Paxinos G, Watson C, Petrides M, Rosa MGP, Tokuno H (2012) The marmoset brain in stereotaxic coordinates. Academic, Amsterdam, London
Perlson E, Maday S, Fu MM, Moughamian AJ, Holzbaur EL (2010) Retrograde axonal transport: pathways to cell death? Trends Neurosci 33:335–344
Pollock A, Hazelton C, Henderson CA, Angilley J, Dhillon B, Langhorne P, Livingstone K, Munro FA, Orr H, Rowe FJ (2011) Interventions for visual field defects in patients with stroke. Cochrane Database Syst Rev 10:CD008388
Pons TP, Kaas JH (1985) Connections of area 2 of somatosensory cortex with the anterior pulvinar and subdivisions of the ventroposterior complex in macaque monkeys. J Comp Neurol 240:16–36
Purushothaman G, Marion R, Li K, Casagrande VA (2012) Gating and control of primary visual cortex by pulvinar. Nat Neurosci 15:905–912
Ratliff WA, Mervis RF, Citron BA, Schwartz B, Rubovitch V, Schreiber S, Pick CG (2020) Effect of mild blast-induced TBI on dendritic architecture of the cortex and hippocampus in the mouse. Sci Rep 10:2206
Rodman HR, Gross CG, Albright TD (1990) Afferent basis of visual response properties in area MT of the macaque. II. Effects of superior colliculus removal. J Neurosci 10:1154–1164
Rosa MGP, Tweedale R, Elston GN (2000) Visual responses of neurons in the middle temporal area of new world monkeys after lesions of striate cortex. J Neurosci 20:5552–5563
Royet JP (1991) Stereology: a method for analysing images. Prog Neurobiol 37:433–474
Selemon LD, Begovic A, Rakic P (2009) Selective reduction of neuron number and volume of the mediodorsal nucleus of the thalamus in macaques following irradiation at early gestational ages. J Comp Neurol 515:454–464
Shin KJ, Lee H-J, Park KM (2019) Alterations of individual thalamic nuclei volumes in patients with migraine. J Headache Pain 20:112
Shipp S (2001) Corticopulvinar connections of areas V5, V4, and V3 in the macaque monkey: a dual model of retinal and cortical topographies. J Comp Neurol 439:469–490
Soares JG, Gattass R, Souza AP, Rosa MGP, Fiorani M Jr, Brandão BL (2001) Connectional and neurochemical subdivisions of the pulvinar in Cebus monkeys. Vis Neurosci 18:25–41
Stepniewska I, Kaas JH (1997) Architectonic subdivisions of the inferior pulvinar in New World and Old-World monkeys. Vis Neurosci 14:1043–1060
Stepniewska I, Ql HX, Kaas JH (2000) Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old-World monkeys. Vis Neurosci 17:529–549
Takakuwa N, Isa K, Onoe H, Takahashi J, Isa T (2021) Contribution of the pulvinar and lateral geniculate nucleus to the control of visually guided saccades in blindsight monkeys. J Neurosci 41:1755–1768
Trojanowski JQ, Jacobson S (1997) The morphology and laminar distribution of cortico-pulvinar neurons in the rhesus monkey. Exp Brain Res 28:51–62
Ungerleider LG, Galkin TW, Mishkin M (1983) Visuotopic organisation of projections from striate cortex to inferior and lateral pulvinar in rhesus monkey. J Comp Neurol 217:137–157
Ungerleider LG, Desimone R, Galkin TW, Mishkin M (1984) Subcortical projections of area MT in the macaque. J Comp Neurol 223:368–386
Walker AE (1938) The primate thalamus. University of Chicago Press, Chicago
Warner CE, Goldshmit Y, Bourne JA (2010) Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei. Front Neuroanat 4:8
Warner CE, Kwan WC, Wright D, Johnston LA, Egan GF, Bourne JA (2015) Preservation of vision by the pulvinar following early-life primary visual cortex lesions. Curr Biol 25:424–434
Williams RW, Rakic P (1988) Three-dimensional counting: an accurate and direct method to estimate numbers of cells in sectioned material. J Comp Neurol 278:344–352
Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blumcke I (1996) NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem 44:1167–1171
Yu HH, Chaplin TA, Egan GW, Reser DH, Worthy KH, Rosa MGP (2013) Visually evoked responses in extrastriate area MT after lesions of striate cortex in early life. J Neurosci 33:12479–12489
Yu HH, Atapour N, Chaplin TA, Worthy KH, Rosa MGP (2018) Robust visual responses and normal retinotopy in primate lateral geniculate nucleus following long-term lesions of striate cortex. J Neurosci 38:3955–3970
Acknowledgements
The authors acknowledge the support of the Monash Histology platform, which provided slide scanning services.
Funding
This research was supported by an Australian Government Research Training Program (RTP) Scholarship to JMC, and by grants from the National Health and Medical Research Council (1122220 and 1194206) to MGPR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval
The experiments were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. All procedures were approved by the Monash University Animal Ethics Experimentation Committee, which also monitored the health and well-being of the animals throughout the experiments.
Consent to participate
Written informed consent was obtained from all authors included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chan, J.M., Worthy, K.H., Rosa, M.G.P. et al. Volume reduction without neuronal loss in the primate pulvinar complex following striate cortex lesions. Brain Struct Funct 226, 2417–2430 (2021). https://doi.org/10.1007/s00429-021-02345-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02345-1