Abstract
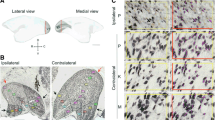
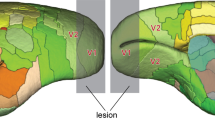
Following lesions of the primary visual cortex (V1), the lateral geniculate nucleus (LGN) undergoes substantial cell loss due to retrograde degeneration. However, visually responsive neurons remain in the degenerated sector of LGN, and these have been implicated in mediation of residual visual capacities that remain within the affected sectors of the visual field. Using immunohistochemistry, we compared the neurochemical characteristics of LGN neurons in V1-lesioned marmoset monkeys (Callithrix jacchus) with those of non-lesioned control animals. We found that GABAergic neurons form approximately 6.5% of the neuronal population in the normal LGN, where most of these cells express the calcium-binding protein parvalbumin. Following long-term V1 lesions in adult monkeys, we observed a marked increase (~ sevenfold) in the proportion of GABA-expressing neurons in the degenerated sector of the LGN, indicating that GABAergic cells are less affected by retrograde degeneration in comparison with magno- and parvocellular projection neurons. In addition, following early postnatal V1 lesions and survival into adulthood, we found widespread expression of GABA in putative projection neurons, even outside the degenerated sectors (lesion projection zones). Our findings show that changes in the ratio of GABAergic neurons in LGN need to be taken into account in the interpretation of the mechanisms of visual abilities that survive V1 lesions in primates.






Similar content being viewed by others
References
Ajina S, Pestilli F, Rokem A, Kennard C, Bridge H (2015) Human blindsight is mediated by an intact geniculo-extrastriate pathway. Elife 4:e08935
Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R (1997) GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res Bull 42:27–37
Atapour N, Worthy KH, Lui LL, Yu HH, Rosa MGP (2017) Neuronal degeneration in the dorsal lateral geniculate nucleus following lesions of primary visual cortex: comparison of young adult and geriatric marmoset monkeys. Brain Struct Funct 222:3283–3293
Atapour N, Majka P, Wolkowicz IH, Malamanova D, Worthy KH, Rosa MGP (2019) Neuronal distribution across the cerebral cortex of the marmoset monkey (Callithrix jacchus). Cereb Cortex 29:3836–3863
Boire D, Théoret H, Ptito M (2002) Stereological evaluation of neurons and glia in the monkey dorsal lateral geniculate nucleus following an early cerebral hemispherectomy. Exp Brain Res 142:208–220
Bourne JA, Warner CE, Rosa MGP (2005) Topographic and laminar maturation of striate cortex in early postnatal marmoset monkeys, as revealed by neurofilament immunohistochemistry. Cereb Cortex 15:740–748
Chaplin TA, Yu HH, Rosa MGP (2013) Representation of the visual field in the primary visual area of the marmoset monkey: magnification factors, point-image size, and proportionality to retinal ganglion cell density. J Comp Neurol 521:1001–1019
Chen C, Bickford ME, Hirsch JA (2016) Untangling the web between eye and brain. Cell 165:20–21
Chiba C, Matsushima O, Muneoka Y, Saito T (1997) Time course of appearance of GABA and GABA receptors during retinal regeneration in the adult newt. Brain Res Dev Brain Res 98:204–210
Cowey A (1974) Atrophy of retinal ganglion cells after removal of striate cortex in a rhesus monkey. Perception 3:257–260
Cowey A, Alexander I, Stoerig P (2011) Transneuronal retrograde degeneration of retinal ganglion cells and optic tract in hemianopic monkeys and humans. Brain 134:2149–2157
Cox CL, Beatty JA (2017) The multifaceted role of inhibitory interneurons in the dorsal lateral geniculate nucleus. Vis Neurosci 34:E017
De A, El-Shamayleh Y, Horwitz GD (2020) Fast and reversible neural inactivation in macaque cortex by optogenetic stimulation of GABAergic neurons. Elife 9:e52658
Dineen JT, Hendrickson AE (1981) Age correlated differences in the amount of retinal degeneration after striate cortex lesions in monkeys. Investig Ophthalmol Vis Sci 21:749–752
Evangelio M, García-Amado M, Clascá F (2018) Thalamocortical projection neuron and interneuron numbers in the visual thalamic nuclei of the adult C57BL/6 mouse. Front Neuroanat 12:27
Fagiolini M, Hensch TK (2000) Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404:183–186
Foeller E, Celikel T, Feldman DE (2005) Inhibitory sharpening of receptive fields contributes to whisker map plasticity in rat somatosensory cortex. J Neurophysiol 94:4387–4400
Fox DM, Goodale MA, Bourne JA (2020) The age-dependent neural substrates of blindsight. Trends Neurosci 43:242–252
Fritsches KA, Rosa MGP (1996) Visuotopic organisation of striate cortex in the marmoset monkey (Callithrix jacchus). J Comp Neurol 372:264–282
Gabbott PL, Somogyi J, Stewart MG, Hámori J (1986) GABA-immunoreactive neurons in the dorsal lateral geniculate nucleus of the rat: characterisation by combined Golgi-impregnation and immunocytochemistry. Exp Brain Res 61:311–322
Gallyas F (1979) Silver staining of myelin by means of physical development. Neurol Res 1:203–209
Goodchild AK, Martin PR (1998) The distribution of calcium-binding proteins in the lateral geniculate nucleus and visual cortex of a New World monkey, the marmoset, Callithrix jacchus. Vis Neurosci 15:625–642
Hagan MA, Rosa MGP, Lui LL (2017) Neural plasticity following lesions of the primate occipital lobe: the marmoset as an animal model for studies of blindsight. Dev Neurobiol 77:314–327
Hagan MA, Chaplin TA, Huxlin KR, Rosa MGP, Lui LL (2020) Altered sensitivity to motion of area MT neurons following long-term V1 lesions. Cereb Cortex 30:451–464
Harel NY, Strittmatter SM (2006) Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci 7:603–616
Hendrickson A, Warner CE, Possin D, Huang J, Kwan WC, Bourne JA (2015) Retrograde transneuronal degeneration in the retina and lateral geniculate nucleus of the V1-lesioned marmoset monkey. Brain Struct Funct 220:351–360
Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF (1998) Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282:1504–1508
Herbin M, Boire D, Théoret H, Ptito M (1999) Transneuronal degeneration of retinal ganglion cells in early hemispherectomized monkeys. NeuroReport 10:1447–1452
Hoyt CS (2003) Visual function in the brain-damaged child. Eye (Lond) 17:369–384
Jones EG, Hendry SH (1989) Differential calcium binding protein immunoreactivity distinguishes classes of relay neurons in monkey thalamic nuclei. Eur J Neurosci 1:222–246
Kinoshita M, Kato R, Isa K, Kobayashi K, Kobayashi K, Onoe H, Isa T (2019) Dissecting the circuit for blindsight to reveal the critical role of pulvinar and superior colliculus. Nat Commun 10:135
Kisvárday ZF, Cowey A, Stoerig P, Somogyi P (1991) Direct and indirect retinal input into degenerated dorsal lateral geniculate nucleus after striate cortical removal in monkey: implications for residual vision. Exp Brain Res 86:271–292
Madarász M, Somogyi G, Somogyi J, Hámori J (1985) Numerical estimation of gamma-aminobutyric acid (GABA)-containing neurons in three thalamic nuclei of the cat: direct GABA immunocytochemistry. Neurosci Lett 61:73–78
Majka P, Chaplin TA, Yu HH, Tolpygo A, Mitra PP, Wójcik DK, Rosa MGP (2016) Towards a comprehensive atlas of cortical connections in a primate brain: mapping tracer injection studies of the common marmoset into a reference digital template. J Comp Neurol 524:2161–2181
Majka P, Bai S, Bakola S, Bednarek S, Chan JM, Jermakow N, Passarelli L, Reser DH, Theodoni P, Worthy KH, Wang XJ, Wójcik DK, Mitra PP, Rosa MGP (2020) Open access resource for cellular-resolution analyses of corticocortical connectivity in the marmoset monkey. Nat Commun 11:1133
Majka P, Bednarek S, Chan JM, Jermakow N, Liu C, Saworska G, Worthy KH, Silva AC, Wójcik DK, Rosa MGP (2021) Histology-based average template of the marmoset cortex with probabilistic localization of cytoarchitectural areas. Neuroimage 226:117625
Missler M, Eins S, Merker HJ, Rothe H, Wolff JR (1993a) Pre- and postnatal development of the primary visual cortex of the common marmoset. I. A changing space for synaptogenesis. J Comp Neurol 333:41–52
Missler M, Wolff A, Merker HJ, Wolff JR (1993b) Pre- and postnatal development of the primary visual cortex of the common marmoset. II. Formation, remodelling, and elimination of synapses as overlapping processes. J Comp Neurol 333:53–67
Montero VM (1986) The interneuronal nature of GABAergic neurons in the lateral geniculate nucleus of the rhesus monkey: a combined HRP and GABA-immunocytochemical study. Exp Brain Res 64:615–622
Montero VM, Zempel J (1986) The proportion and size of GABA-immunoreactive neurons in the magnocellular and parvocellular layers of the lateral geniculate nucleus of the rhesus monkey. Exp Brain Res 62:215–223
Obrietan K, Gao XB, Van Den Pol AN (2002) Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism—a positive feedback circuit in developing neurons. J Neurophysiol 88:1005–1015
Paxinos G, Watson C, Petrides M, Rosa MGP, Tokuno H (2012) The marmoset brain in stereotaxic coordinates. Academic, Amsterdam
Ptito A, Leh SE (2007) Neural substrates of blindsight after hemispherectomy. Neuroscientist 13:506–518
Romaus-Sanjurjo D, Ledo-García R, Fernández-López B, Hanslik K, Morgan JR, Barreiro-Iglesias A, Rodicio MC (2018) GABA promotes survival and axonal regeneration in identifiable descending neurons after spinal cord injury in larval lampreys. Cell Death Dis 9:663
Rosa MGP, Tweedale R, Elston GN (2000) Visual responses of neurons in the middle temporal area of new world monkeys after lesions of striate cortex. J Neurosci 20:5552–5563
Sawiak SJ, Shiba Y, Oikonomidis L, Windle CP, Santangelo AM, Grydeland H, Cockcroft G, Bullmore ET, Roberts AC (2018) Trajectories and milestones of cortical and subcortical development of the marmoset brain from infancy to adulthood. Cereb Cortex 28:4440–4453
Schultz-Darken N, Braun KM, Emborg ME (2016) Neurobehavioral development of common marmoset monkeys. Dev Psychobiol 58:141–158
Seabrook TA, Krahe TE, Govindaiah G, Guido W (2013) Interneurons in the mouse visual thalamus maintain a high degree of retinal convergence throughout postnatal development. Neural Dev 8:1–7
Shulga A, Rivera C (2013) Interplay between thyroxin, BDNF and GABA in injured neurons. Neuroscience 239:241–252
Solomon SG, Rosa MGP (2014) A simpler primate brain: the visual system of the marmoset monkey. Front Neural Circuits 8:96
Spatz WB (1989) Loss of ocular dominance columns with maturity in the monkey, Callithrix jacchus. Brain Res 488:376–380
Stichel CC, Singer W, Heizmann CW (1988) Light and electron microscopic immunocytochemical localization of parvalbumin in the dorsal lateral geniculate nucleus of the cat: evidence for coexistence with GABA. J Comp Neurol 268:29–37
Takács J, Hámori J, Silakov V (1991) GABA-containing neuronal processes in normal and cortically deafferented dorsal lateral geniculate nucleus of the cat: an immunogold and quantitative EM study. Exp Brain Res 83:562–574
Takakuwa N, Isa K, Onoe H, Takahashi J, Isa T (2021) Contribution of the pulvinar and lateral geniculate nucleus to the control of visually guided saccades in blindsight monkeys. J Neurosci 41:1755–1768
Tamietto M, Morrone MC (2016) Visual plasticity: blindsight bridges anatomy and function in the visual system. Curr Biol 26:R70–R73
Tumosa N, Tieman SB, Tieman DG (1989) Binocular competition affects the pattern and intensity of ocular activation columns in the visual cortex of cats. Vis Neurosci 2:391–407
Wang DD, Kriegstein AR (2008) GABA regulates excitatory synapse formation in the neocortex via NMDA receptor activation. J Neurosci 28:5547–5558
Warner CE, Goldshmit Y, Bourne JA (2010) Retinal afferents synapse with relay cells targeting the middle temporal area in the pulvinar and lateral geniculate nuclei. Front Neuroanat 4:8
Warner CE, Kwan WC, Wright D, Johnston LA, Egan GF, Bourne JA (2015) Preservation of vision by the pulvinar following early-life primary visual cortex lesions. Curr Biol 25:424–434
Weller RE, Kaas JH (1989) Parameters affecting the loss of ganglion cells of the retina following ablations of striate cortex in primates. Vis Neurosci 3:327–349
White AJ, Wilder HD, Goodchild AK, Sefton AJ, Martin PR (1998) Segregation of receptive field properties in the lateral geniculate nucleus of a New-World monkey, the marmoset Callithrix jacchus. J Neurophysiol 80:2063–2076
Yan YH, Winarto A, Mansjoer I, Hendrickson A (1996) Parvalbumin, calbindin, and calretinin mark distinct pathways during development of monkey dorsal lateral geniculate nucleus. J Neurobiol 31:189–209
Yu HH, Chaplin TA, Egan GW, Reser DH, Worthy KH, Rosa MGP (2013) Visually evoked responses in extrastriate area MT after lesions of striate cortex in early life. J Neurosci 33:12479–12489
Yu HH, Chaplin TA, Rosa MGP (2015) Representation of central and peripheral vision in the primate cerebral cortex: insights from studies of the marmoset brain. Neurosci Res 93:47–61
Yu HH, Atapour N, Chaplin TA, Worthy KH, Rosa MGP (2018) Robust visual responses and normal retinotopy in primate lateral geniculate nucleus following long-term lesions of striate cortex. J Neurosci 38:3955–3970
Zepeda A, Sengpiel F, Guagnelli MA, Vaca L, Arias C (2004) Functional reorganization of visual cortex maps after ischemic lesions is accompanied by changes in expression of cytoskeletal proteins and NMDA and GABA(A) receptor subunits. J Neurosci 24:1812–1821
Acknowledgements
The authors acknowledge the contributions of Monash Micro Imaging (MMI) facility for providing training and support for confocal imaging.
Funding
Funded by grants from the National Health and Medical Research Council (1122220 and 1194206).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval
The experiments were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. All procedures were approved by the Monash University Animal Ethics Experimentation Committee, which also monitored the health and wellbeing of the animals throughout the experiments.
Consent to participate
Written informed consent was obtained from all authors included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Atapour, N., Worthy, K.H. & Rosa, M.G.P. Neurochemical changes in the primate lateral geniculate nucleus following lesions of striate cortex in infancy and adulthood: implications for residual vision and blindsight. Brain Struct Funct 226, 2763–2775 (2021). https://doi.org/10.1007/s00429-021-02257-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02257-0