Abstract
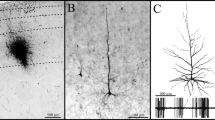
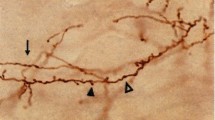
Emerging appreciation for the hyperdirect pathway (HDP) as an important cortical glutamatergic input to the subthalamic nucleus (STN) has motivated a wide range of recent investigations on its role in motor control, as well as the mechanisms of subthalamic deep brain stimulation (DBS). However, the pathway anatomy and terminal arbor morphometry by which the HDP links cortical and subthalamic activity are incompletely understood. One critical hindrance to advancing understanding is the lack of anatomically detailed population models which can help explain how HDP pathway anatomy and neuronal biophysics give rise to spatiotemporal patterns of stimulus–response activity observed in vivo. Therefore, the goal of this study was to establish a population model of motor HDP axons through application of generative algorithms constrained by recent histology and imaging data. The products of this effort include a de novo macaque brain atlas, detailed statistical analysis of histological reconstructions of macaque motor HDP axons, and the generation of 10,000 morphometrically constrained synthetic motor HDP axons. The synthetic HDP axons exhibited a 3.8% mean error with respect to parametric distributions of the fiber target volume, total length, number of bifurcations, bifurcation angles, meander angles, and segment lengths measured in BDA-labeled HDP axon reconstructions. As such, this large population of synthetic motor HDP axons represents an anatomically based foundation for biophysical simulations that can be coupled to electrophysiological and/or behavioral measurements, with the goal of better understanding the role of the HDP in motor system activity.








Similar content being viewed by others
Data availability
The cynomolgus macaque brain atlas (‘stl’ and labeled ‘nii’ format) and HDP fiber models (‘swc’ format), as well as supporting scripts, are available on GitHub (https://github.com/ClaytonBingham/MacaqueHyperdirectPathway). The Attracted Growth Cone Search Method, written in Python (v3.7), is also available for download (https://github.com/ClaytonBingham/AttractedGrowthConeSearch).
Code availability
Code is available upon request.
References
Abdellah M, Hernando J, Eilemann S, Lapere S, Antille N, Markram H, Schürmann F (2018) NeuroMorphoVis: a collaborative framework for analysis and visualization of neuronal morphology skeletons reconstructed from microscopy stacks. Bioinformatics 34(13):i574–i582
Alexander GE, Crutcher MD, DeLong MR (1991) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85:119–146
Anderson R, Farokhniaee A, Gunalan K, Howell B, McIntyre C (2018) Action potential initiation, propagation, and cortical invasion in the hyperdirect pathway during subthalamic deep brain stimulation. Brain Stimul 11(5):1140–1150
Bingham CS, Bouteiller JM, Song D, Berger TW (2018) Graph-based models of cortical axons for the prediction of neuronal response to extracellular electrical stimulation. 2018 40th annual international conference of the IEEE engineering in medicine and biology society (EMBC).
Bingham C, Mergenthal A, Bouteiller J-M, Song D, Lazzi G, Berger T (2020) ROOTS: an algorithm to generate biologically realistic cortical axons and an application to electroceutical modeling. Front Comput Neurosci 14:13
Bower KL, McIntyre CC (2020) Deep brain stimulation of terminating axons. Brain Stimul 13(6):1863–1870
Chakravarty MMSF, Collins DL, Paxinos G, Huang XF, Petrides M, Toga AW (2008) Digital atlas of the monkey brain in stereotactic co-ordinates. In the rhesus monkey brain in stereotactic coordinates. Academic
Coudé D, Parent A, Parent M (2018) Single-axon tracing of the corticosubthalamic hyperdirect pathway in primates. Brain Struct Funct 223(9):3959–3973
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29(3):162–173
Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M (2009) Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul 2(4):201–207
Feng L, Zhao T, Kim J (2015). neuTube 1.0: a new design for efficient neuron reconstruction software based on The SWC format. eNeuro.
Fischl B (2012) FreeSurfer. Neuroimage 62(2):774–781
Fox CH, Johnson FB, Whiting J, Roller PP (1985) Formaldehyde fixation. J Histochem Cytochem 33(8):845–853
Frey S, Pandya DN, Chakravarty MM, Bailey L, Petrides M, Collins DL (2011) An MRI based average macaque monkey stereotaxic atlas and space (MNI monkey space). Neuroimage 55(4):1435–1442
Gunalan K, McIntyre CC (2020) Biophysical reconstruction of the signal conduction underlying short-latency cortical evoked potentials generated by subthalamic deep brain stimulation. Clin Neurophysiol 131(2):542–547
Gusnard D, Kirschner RH (1977) Cell and organelle shrinkage during preparation for scanning electron microscopy: effects of fixation, dehydration and critical point drying. J Microsc 110(1):51–57
Hahn PJ, McIntyre CC (2010) Modeling shifts in the rate and pattern of subthalamopallidal network activity during deep brain stimulation. J Comput Neurosci 28(3):425–441
Haynes WI, Haber SN (2013) The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci 33(11):4804–4814
Johnson LA, Wang J, Nebeck SD, Zhang J, Johnson MD, Vitek JL (2020) Direct activation of primary motor cortex during subthalamic but not pallidal deep brain stimulation. J Neurosci 40(10):2166–2177
Kazhdan M, Hoppe H (2013) Screened poisson surface reconstruction. ACM Trans Graphics (ToG) 32(3):29
Kita T, Kita H (2012) The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci 32(17):5990–5999
Kumaravelu K, Oza CS, Behrend CE, Grill WM (2018) Model-based deconstruction of cortical evoked potentials generated by subthalamic nucleus deep brain stimulation. J Neurophysiol 120(2):662–680
Maling N, Lempka SF, Blumenfeld Z, Bronte-Stewart H, McIntyre CC (2018) Biophysical basis of subthalamic local field potentials recorded from deep brain stimulation electrodes. J Neurophysiol 120(4):1932–1944
Marder E, Taylor AL (2011) Multiple models to capture the variability in biological neurons and networks. Nat Neurosci 14(2):133–138
Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, Kahou GA (2015) Reconstruction and simulation of neocortical microcircuitry. Cell 132(2):456–492
Mathai A, Ma Y, Paré JF, Villalba RM, Wichmann T, Smith Y (2015) Reduced cortical innervation of the subthalamic nucleus in MPTP-treated Parkinsonian monkeys. Brain 138(4):946–962
Miocinovic S, de Hemptinne C, Chen W, Isbaine F, Willie JT, Ostrem JL, Starr PA (2018) Cortical potentials evoked by subthalamic stimulation demonstrate a short latency hyperdirect pathway in humans. J Neurosci 38(43):9129–9141
Morales-Napoles O, Cooke RM, Kurowicka D (2010) About the number of vines and regular vines on n nodes. Delft University of Technology
Nambu A, Takada M, Inase M, Tokuno H (1996) Dual somatotopical representations in the primate subthalamic nucleus: evidence for ordered but reversed body-map transformations from the primary motor cortex and the supplementary motor area. J Neurosci 16(8):2671–2683
Nambu A, Tokuno H, Takada M (2002) Functional significance of the cortico–subthalamo–pallidal ‘hyperdirect’ pathway. Neurosci Res 43(2):111–117
Nelson AB, Kreitzer AC (2014) Reassessing models of basal ganglia function and dysfunction. Annu Rev Neurosci 37:117–135
Opitz A, Legon W, Rowlands A, Bickel K, Paulus W, Tyler WJ (2013) Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage 81:253–264
Ourselin S, Roche A, Subsol G, Pennec X, Ayache N (2001) Reconstructing a 3D structure from serial histological sections. Image vis Comput 19(1–2):25–31
Parent A, Hazrati LN (1995) Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res 20(1):91–127
Petersen MV, Mlakar J, Haber SN, Parent M, Smith Y, Strick PL, McIntyre CC (2019) Holographic reconstruction of axonal pathways in the human brain. Neuron 104(6):1056–1064
Plantinga BR, Temel Y, Duchin Y, Uludağ K, Patriat R, Roebroeck A, Harel N (2018) Individualized parcellation of the subthalamic nucleus in patients with Parkinson’s disease with 7T MRI. Neuroimage 168:403–411
Polyakova Z, Chiken S, Hatanaka N, Nambu A (2020) Cortical control of subthalamic neuronal activity through the hyperdirect and indirect pathways in monkeys. J Neurosci 40(39):7451–7463
Silverman BW (2018) Density estimation for statistics and data analysis. Routledge
Wearne SL, Rodriguez A, Ehlenberger DB, Rocher AB, Henderson SC, Hof PR (2005) New techniques for imaging, digitization and analysis of three-dimensional neural morphology on multiple scales. Neuroscience 136(3):661–680
Yu C, Cassar IR, Sambangi J, Grill WM (2020) Frequency-specific optogenetic deep brain stimulation of subthalamic nucleus improves Parkinsonian motor behaviors. J Neurosci 40(22):4323–4334
Acknowledgements
The authors thank Kelsey Bower, Sohail Noor, and Mevlut Yalaz for reviewing and suggesting edits to the study and manuscript ahead of submission. We also acknowledge Constantin Delmas for assisting in the organization and description of the histology data.
Funding
National Institutes of Health (R01 NS105690; R01 NS086100), Canadian Institutes of Health Research (CIHR MOP-153068).
Author information
Authors and Affiliations
Contributions
Conceptualization: CSB and CCM; Methodology: CSB and CCM; Formal analysis and investigation: CSB and CCM; Writing—original draft preparation: CSB and CCM; Writing—review and editing: CSB, CCM, and MP; Funding acquisition: CCM and MP; Resources: CCM and MP; Supervision: CCM.
Corresponding author
Ethics declarations
Conflict of interest
CCM is a paid consultant for Boston Scientific Neuromodulation, receives royalties from Hologram Consultants, Neuros Medical, Qr8 Health, and is a shareholder in the following companies: Hologram Consultants, Surgical Information Sciences, CereGate, Autonomic Technologies, Cardionomic, Enspire DBS.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bingham, C.S., Parent, M. & McIntyre, C.C. Histology-driven model of the macaque motor hyperdirect pathway. Brain Struct Funct 226, 2087–2097 (2021). https://doi.org/10.1007/s00429-021-02307-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02307-7