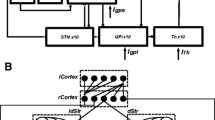
Abstract
Deep brain stimulation (DBS) of the subthlamic nucleus (STN) represents an effective treatment for medically refractory Parkinson’s disease; however, understanding of its effects on basal ganglia network activity remains limited. We constructed a computational model of the subthalamopallidal network, trained it to fit in vivo recordings from parkinsonian monkeys, and evaluated its response to STN DBS. The network model was created with synaptically connected single compartment biophysical models of STN and pallidal neurons, and stochastically defined inputs driven by cortical beta rhythms. A least mean square error training algorithm was developed to parameterize network connections and minimize error when compared to experimental spike and burst rates in the parkinsonian condition. The output of the trained network was then compared to experimental data not used in the training process. We found that reducing the influence of the cortical beta input on the model generated activity that agreed well with recordings from normal monkeys. Further, during STN DBS in the parkinsonian condition the simulations reproduced the reduction in GPi bursting found in existing experimental data. The model also provided the opportunity to greatly expand analysis of GPi bursting activity, generating three major predictions. First, its reduction was proportional to the volume of STN activated by DBS. Second, GPi bursting decreased in a stimulation frequency dependent manner, saturating at values consistent with clinically therapeutic DBS. And third, ablating STN neurons, reported to generate similar therapeutic outcomes as STN DBS, also reduced GPi bursting. Our theoretical analysis of stimulation induced network activity suggests that regularization of GPi firing is dependent on the volume of STN tissue activated and a threshold level of burst reduction may be necessary for therapeutic effect.










Similar content being viewed by others
References
Alvarez, L., Macias, R., Pavon, N., Lopez, G., Rodriguez-Oroz, M. C., Rodriguez, R., et al. (2009). Therapeutic efficacy of unilateral subthalamotomy in Parkinson's disease: results in 89 patients followed for up to 36 months. Journal of Neurology, Neurosurgery and Psychiatry, 80, 979–985.
Bekar, L., Libionka, W., Tian, G. F., Xu, Q., Torres, A., Wang, X., et al. (2008). Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nature Medicine, 14, 75–80.
Bergman, H., Wichmann, T., Karmon, B., & DeLong, M. R. (1994). The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. Journal of Neurophysiology, 72, 507–520.
Bevan, M. D., Magill, P. J., Terman, D., Bolam, J. P., & Wilson, C. J. (2002). Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends in Neurosciences, 25(10), 525–31.
Birdno, M. J., Kuncel, A. M., Dorval, A. D., Turner, D. A., & Grill, W. M. (2008). Tremor varies as a function of the temporal regularity of deep brain stimulation. NeuroReport, 19, 599–602.
Brown, P. (2000). Cortical drives to human muscle: the Piper and related rhythms. Progress in Neurobiology, 60, 97–108.
Brown, P., & Williams, D. (2005). Basal ganglia local field potential activity: character and functional significance in the human. Clinical Neurophysiology, 116, 2510–2519.
Brown, P., Oliviero, A., Mazzone, P., Insola, A., Tonali, P., & Di Lazzaro, V. (2001). Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. Journal of Neuroscience, 21, 1033–1038.
Butson, C. R., Cooper, S. E., Henderson, J. M., & McIntyre, C. C. (2007). Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage, 34, 661–670.
Carnevale, N. T., Hines, M. L. (2005). The Neuron book. Cambridge University Press.
Cooper, A. J., & Stanford, I. M. (2000). Electrophysiological and morphological characteristics of three subtypes of rat globus pallidus neurone in vitro. Journal of Physiology, 527(Pt 2), 291–304.
Courtemanche, R., Fujii, N., & Graybiel, A. M. (2003). Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. Journal of Neuroscience, 23, 11741–11752.
Dejean, C., Gross, C. E., Bioulac, B., & Boraud, T. (2008). Dynamic changes in the cortex-basal ganglia network after dopamine depletion in the rat. Journal of Neurophysiology, 100, 385–396.
Destexhe, A., Mainen, Z. F., & Sejnowski, T. J. (1994a). An efficient method for computing synaptic conductances based on a kinetic model of receptor binding. Neural Computation, 6, 10–14.
Destexhe, A., Mainen, Z. F., & Sejnowski, T. J. (1994b). Synthesis of models for excitable membranes, synaptic transmission and neuromodulation using a common kinetic formalism. Journal of Computational Neuroscience, 1, 195–230.
Feng, X. J., Greenwald, B., Rabitz, H., Shea-Brown, E., & Kosut, R. (2007). Toward closed-loop optimization of deep brain stimulation for Parkinson's disease: concepts and lessons from a computational model. Journal of Neural Engineering, 4, L14–L21.
Galvan, A., & Wichmann, T. (2008). Pathophysiology of parkinsonism. Clinical Neurophysiology, 119, 1459–1474.
Goldberg, J. A., Kats, S. S., & Jaeger, D. (2003). Globus pallidus discharge is coincident with striatal activity during global slow wave activity in the rat. Journal of Neuroscience, 23, 10058–10063.
Goldberg, J. A., Rokni, U., Boraud, T., Vaadia, E., & Bergman, H. (2004). Spike synchronization in the cortex/basal-ganglia networks of Parkinsonian primates reflects global dynamics of the local field potentials. Journal of Neuroscience, 24, 6003–6010.
Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M., & Deisseroth, K. (2009). Optical deconstruction of parkinsonian neural circuitry. Science, 324, 354–359.
Guo, Y., Rubin, J. E., McIntyre, C. C., Vitek, J. L., & Terman, D. (2008). Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. Journal of Neurophysiology, 99, 1477–1492.
Hahn, P. J., Russo, G. S., Hashimoto, T., Miocinovic, S., Xu, W., McIntyre, C. C., et al. (2008). Pallidal burst activity during therapeutic deep brain stimulation. Experimental Neurology, 211, 243–251.
Hammond, C., Bergman, H., & Brown, P. (2007). Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends in Neurosciences, 30, 357–364.
Hardman, C. D., Henderson, J. M., Finkelstein, D. I., Horne, M. K., Paxinos, G., & Halliday, G. M. (2002). Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. Journal of Comparative Neurology, 445, 238–255.
Hashimoto, T., Elder, C. M., Okun, M. S., Patrick, S. K., & Vitek, J. L. (2003). Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. Journal of Neuroscience, 23, 1916–1923.
Humphries, M. D., Stewart, R. D., & Gurney, K. N. (2006). A physiologically plausible model of action selection and oscillatory activity in the basal ganglia. Journal of Neuroscience, 26, 12921–12942.
Kuhn, A. A., Trottenberg, T., Kivi, A., Kupsch, A., Schneider, G. H., & Brown, P. (2005). The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson's disease. Experimental Neurology, 194, 212–220.
Leblois, A., Boraud, T., Meissner, W., Bergman, H., & Hansel, D. (2006). Competition between feedback loops underlies normal and pathological dynamics in the basal ganglia. Journal of Neuroscience, 26, 3567–3583.
Li, S., Arbuthnott, G. W., Jutras, M. J., Goldberg, J. A., & Jaeger, D. (2007). Resonant antidromic cortical circuit activation as a consequence of high-frequency subthalamic deep-brain stimulation. Journal of Neurophysiology, 98, 3525–3537.
Magnin, M., Morel, A., & Jeanmonod, D. (2000). Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience, 96, 549–564.
Maks, C. B., Butson, C. R., Walter, B. L., Vitek, J. L., & McIntyre, C. C. (2009). Deep brain stimulation activation volumes and their association with neurophysiological mapping and therapeutic outcomes. Journal of Neurology, Neurosurgery and Psychiatry, 80, 659–666.
McIntyre, C. C., & Hahn, P. J. (2010). Network perspectives on the mechanisms of deep brain stimulation. Neurobiology of Disease. doi:10.1016/j.nbd.2009.09.022.
Mink, J. W. (1996). The basal ganglia: focused selection and inhibition of competing motor programs. Progress in Neurobiology, 50, 381–425.
Mink, J. W., & Thach, W. T. (1993). Basal ganglia intrinsic circuits and their role in behavior. Current Opinion in Neurobiology, 3, 950–957.
Miocinovic, S., Parent, M., Butson, C. R., Hahn, P. J., Russo, G. S., Vitek, J. L., et al. (2006). Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. Journal of Neurophysiology, 96, 1569–1580.
Modolo, J., Henry, J., & Beuter, A. (2008). Dynamics of the Subthalamo-pallidal Complex in Parkinson's Disease During Deep Brain Stimulation. Journal of biological physics, 34, 251–266.
Montgomery, E. B., Jr., & Baker, K. B. (2000). Mechanisms of deep brain stimulation and future technical developments. Neurological Research, 22, 259–266.
Nambu, A., & Llinas, R. (1994). Electrophysiology of globus pallidus neurons in vitro. Journal of Neurophysiology, 72, 1127–1139.
Nandhagopal, R., McKeown, M. J., & Stoessl, A. J. (2008). Functional imaging in Parkinson disease. Neurology, 70, 1478–1488.
Nini, A., Feingold, A., Slovin, H., & Bergman, H. (1995). Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. Journal of Neurophysiology, 74, 1800–1805.
Otsuka, T., Abe, T., Tsukagawa, T., & Song, W. J. (2004). Conductance-based model of the voltage-dependent generation of a plateau potential in subthalamic neurons. Journal of Neurophysiology, 92, 255–264.
Phillips, M. D., Baker, K. B., Lowe, M. J., Tkach, J. A., Cooper, S. E., Kopell, B. H., et al. (2006). Parkinson disease: pattern of functional MR imaging activation during deep brain stimulation of subthalamic nucleus–initial experience. Radiology, 239, 209–216.
Plenz, D., & Kital, S. T. (1999). A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature, 400, 677–682.
Press, W. H., Teukolsky, S. A., Vetterling, W. T., Flannery, B. P. (1992). Numerical recipes in C, The art of scientific computing, 2nd edition. Cambridge University Press.
Raz, A., Vaadia, E., & Bergman, H. (2000). Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine vervet model of parkinsonism. Journal of Neuroscience, 20, 8559–8571.
Rizzone, M., Lanotte, M., Bergamasco, B., Tavella, A., Torre, E., Faccani, G., et al. (2001). Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: effects of variation in stimulation parameters. Journal of Neurology, Neurosurgery and Psychiatry, 71, 215–219.
Rubchinsky, L. L., Kopell, N., & Sigvardt, K. A. (2003). Modeling facilitation and inhibition of competing motor programs in basal ganglia subthalamic nucleus-pallidal circuits. Proc Natl Acad Sci USA, 100, 14427–14432.
Rubin, J. E., & Terman, D. (2004). High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. Journal of Computational Neuroscience, 16, 211–235.
Sharott, A., Magill, P. J., Bolam, J. P., & Brown, P. (2005a). Directional analysis of coherent oscillatory field potentials in the cerebral cortex and basal ganglia of the rat. Journal of Physiology, 562, 951–963.
Sharott, A., Magill, P. J., Harnack, D., Kupsch, A., Meissner, W., & Brown, P. (2005b). Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. European Journal of Neuroscience, 21, 1413–1422.
Shils, J. L., Mei, L. Z., & Arle, J. E. (2008). Modeling parkinsonian circuitry and the DBS electrode. II. Evaluation of a computer simulation model of the basal ganglia with and without subthalamic nucleus stimulation. Stereotactic and Functional Neurosurgery, 86, 16–29.
Soares, J., Kliem, M. A., Betarbet, R., Greenamyre, J. T., Yamamoto, B., & Wichmann, T. (2004). Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. Journal of Neuroscience, 24, 6417–6426.
Tass, P. A. (2003). A model of desynchronizing deep brain stimulation with a demand-controlled coordinated reset of neural subpopulations. Biological Cybernetics, 89, 81–88.
Terman, D., Rubin, J. E., Yew, A. C., & Wilson, C. J. (2002). Activity patterns in a model for the subthalamopallidal network of the basal ganglia. Journal of Neuroscience, 22, 2963–2976.
Wichmann, T., & Soares, J. (2006). Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. Journal of Neurophysiology, 95, 2120–2133.
Wichmann, T., Bergman, H., Starr, P. A., Subramanian, T., Watts, R. L., & DeLong, M. R. (1999). Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Experimental Brain Research, 125, 397–409.
Xu, W., Russo, G. S., Hashimoto, T., Zhang, J., & Vitek, J. L. (2008). Subthalamic nucleus stimulation modulates thalamic neuronal activity. J Neurosci, 28, 11916–11924.
Acknowledgments
This project was supported by the National Institutes of Health (R01 NS047388). The authors thank Jerrold Vitek, Takao Hashimoto, Weidong Xu, and Gary Russo for their contributions to the collection and analysis of the experimental data used in this study. In addition, the authors thank Dongchul Lee and Svjetlana Miocinovic for their contributions to the model development.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: David Terman
Appendix
Appendix
The network model was composed of single compartment, conductance based neuron models. The membrane voltage of each single cell was evaluated in the NEURON simulation environment (v5.8). Action potentials were detected and postsynaptic cells were notified following a set delay that a presynaptic event occurred. Source code for the published network model is available on the NeuronDB database.
1.1 Membrane currents
The active currents included in the single cell models followed a basic Hodgkin-Huxley paradigm. The individual channel kinetics were based on the formulation described in Otsuka et al. (2004), and parameters were defined to reproduce the in vitro firing properties of isolated rodent neurons (Otsuka et al. 2004; Nambu and Llinas 1994; Cooper and Stanford 2000). Corresponding intracellular current-clamp and voltage-clamp recordings from primates do not exist, so we used the next most appropriate animal model. However, we believe this is a relatively minor issue, as isolated neurons of the basal ganglia at are typically tonic firers with highly consistent pacemaker activity. We propose that the vast majority of the modulation seen in vivo is the result of network interactions, and as such we elected to concentrate on the network influence of neural activity rather than the possible nuances of interspecies channel kinetic differences. The maximum conductances for the STN and pallidal models are given in Table 5.
The gating variables follow first order dynamics of the form
where \( {x_\infty }\left( {{V_m}} \right) = {\left[ {1 + \exp \left( {\frac{{{V_m} - {\theta_{\infty, x}}}}{{{\sigma_{\infty, x}}}}} \right)} \right]^{ - 1}} \)and
Tables 6 and 7 list the values of the parameters for the STN and pallidal model kinetics. The steady state activation value for the calcium dependent potassium current (r ∞) and the inactivation value for L-type calcium current (d 2,∞) were functions of calcium level (Cai), rather than membrane voltage.
1.2 Calcium dynamics
Calcium level (Cai) follows the equation
The variable Cai was not meant to be a uniform intracellular calcium concentration. Rather, it was the calcium available at or near membrane bound potassium channels. Cai was increased by calcium currents (negative inward by convention) and decreased by calcium pumps. The constant ε Ca represents the combined effects of intracellular calcium buffering mechanisms and cellular geometry and F is Faraday’s constant. The constant kCa is the calcium pump rate. For all simulations ε Ca = 337.1 and k Ca = .2/ ε Ca .
1.3 Synaptic currents
Synaptic currents were modeled by the equation \( {I_{syn}} = R\left( {{V_m} - {E_{rev}}} \right) \) where R is an activation level and E rev is the reversal potential (0 for AMPA and −80 for GABA synapses) (Destexhe et al. 1994a,b). Each synapse received events from its presynaptic cells. The conductance of the synapse increased exponentially toward the maximum conductance (gSrcTar set for each pathway) following a presynaptic event, and decreased exponentially following a set time (t rise ) during which no presynaptic events occurred. If multiple events occurred within a short period of time, R did not decay back to 0 and therefore reached levels closer to gSrcTar. The synapse could be thought of as having two states, “ON”, where \( \frac{{dR}}{{dt}} = \alpha \left( {{R_{\max }} - R} \right) \) and “OFF”, where \( \frac{{dR}}{{dt}} = - \beta R \) and the initial condition was taken as R at the time of the last spike. This scheme was implemented as a continuous function dependent on the time of the last spike and the count of spikes occurring within a recent interval corresponding to the rise time of the synaptic current (t rise = .3).
1.4 Affects of DA level
Changing DA level from the MPTP state to the Normal state effected three parameters for each pathway in the network model, namely psyn and mean background interspike interval for input pathways, as well as scaling of maximal conductance for most BG pathways. The value of psyn was 0.25 in the MPTP state and 0.05 in the Normal state, as described in Methods. The mean interspike interval increased from 75 in the MPTP state to 100 in the Normal state in the CtxSTN and StrGPe pathways. The mean interspike interval decreased from 150 in the MPTP state to 100 in the Normal state in the StrGPi pathway. DA level has also been modeled as a scaling factor multiplying maximal synaptic conductances in BG (Humphries et al. 2006). Accordingly, a multiplicative factor for maximal synaptic conductances decreased from 1.25 in the MPTP state to 1.0 in the Normal state for the CtxSTN, StrGPe, GPeSTN and GPeGPi pathways and increased from 1.25 to 2.0 in the STNGPe pathway. These changes enabled the model to more closely follow the cortical beta rhythm in the low DA state.
Rights and permissions
About this article
Cite this article
Hahn, P.J., McIntyre, C.C. Modeling shifts in the rate and pattern of subthalamopallidal network activity during deep brain stimulation. J Comput Neurosci 28, 425–441 (2010). https://doi.org/10.1007/s10827-010-0225-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-010-0225-8