Abstract
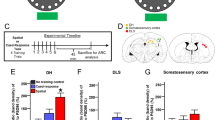
The ventral midline thalamus contributes to hippocampo-cortical interactions supporting systems-level consolidation of memories. Recent hippocampus-dependent memories rely on hippocampal connectivity remodeling. Remote memories are underpinned by neocortical connectivity remodeling. After a ventral midline thalamus lesion, recent spatial memories are formed normally but do not last. Why these memories do not endure after the lesion is unknown. We hypothesized that a lesion could interfere with hippocampal and/or neocortical connectivity remodeling. To test this hypothesis, in a first experiment male rats were subjected to lesion of the reuniens and rhomboid (ReRh) nuclei, trained in a water maze, and tested in a probe trial 5 or 25 days post-acquisition. Dendritic spines were counted in the dorsal hippocampus and medial prefrontal cortex. Spatial learning resulted in a significant increase of mushroom spines in region CA1. This modification persisted between 5 and 25 days post-acquisition in Sham rats, not in rats with ReRh lesion. Furthermore, 25 days after acquisition, the number of mushroom spines in the anterior cingulate cortex (ACC) had undergone a dramatic increase in Sham rats; ReRh lesion prevented this gain. In a second experiment, the increase of c-Fos expression in CA1 accompanying memory retrieval was not affected by the lesion, be it for recent or remote memory. However, in the ACC, the lesion had reduced the retrieval-triggered c-Fos expression observed 25 days post-acquisition. These observations suggest that a ReRh lesion might disrupt spatial remote memory formation by preventing persistence of early remodeled hippocampal connectivity, and spinogenesis in the ACC.




Similar content being viewed by others
References
Abate G, Colazingari S, Accoto A, Conversi D, Bevilacqua A (2018) Dendritic spine density and EphrinB2 levels of hippocampal and anterior cingulate cortex neurons increase sequentially during formation of recent and remote fear memory in the mouse. Behav Brain Res 344:120–131
Aceti M, Vetere G, Novembre G, Restivo L, Ammassari-Teule M (2015) Progression of activity and structural changes in the anterior cingulate cortex during remote memory formation. Neurobiol Learn Mem 123:67–71
Ali M, Cholvin T, Muller MA, Cosquer B, Kelche C, Cassel JC, Pereira de Vasconcelos A (2017) Environmental enrichment enhances systems-level consolidation of a spatial memory after lesions of the ventral midline thalamus. Neurobiol Learn Mem 141:108–123
Beltrán-Campos V, Prado-Alcalá RA, León-Jacinto U, Aguilar-Vázquez A, Quirarte GL, Ramírez-Amaya V, Díaz-Cintra S (2011) Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain Res 1369:119–130
Bourne J, Harris KM (2007) Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17:381–386
Cassel JC, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP (2013) The reuniens and rhomboid nuclei: neuroanatomy, electrophysiological characteristics and behavioral implications. Prog Neurobiol 111:34–52
Cholvin T, Loureiro M, Cassel R, Cosquer B, Geiger K, De Sa Nogueira D, Raingard H, Robelin L, Kelche C, Pereira de Vasconcelos A, Cassel JC (2013) The ventral midline thalamus contributes to strategy shifting in a memory task requiring both prefrontal cortical and hippocampal functions. J Neurosci 33:8772–8783
Cole CJ, Mercaldo V, Restivo L, Yiu AP, Sekeres MJ, Han JH, Vetere G, Pekar T, Ross PJ, Neve RL, Frankland PW, Josselyn SA (2012) MEF2 negatively regulates learning-induced structural plasticity and memory formation. Nat Neurosci 15:1255–1264
Dolleman-van der Weel MJ, Morris RG, Witter MP (2009) Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct Funct 213(3):329–342
Frankland PW, Bontempi B (2005) The organization of recent and remote memories. Nat Rev Neurosci 6:119–130
González-Ramírez MM, Velázquez-Zamora DA, Olvera-Cortés ME, González-Burgos I (2014) Changes in the plastic properties of hippocampal dendritic spines underlie the attenuation of place learning in healthy aged rats. Neurobiol Learn Mem 109:94–103
Griffin AL (2015) Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Front Syst Neurosci 9:29
Groenewegen HJ, Witter MP (2004) Thalamus. In: Paxinos G (ed) The Rat Nervous System. 3th ed
Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B (1988) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96:857–881
Hallock HL, Wang A, Griffin AL (2016) Ventral midline thalamus is critical for hippocampal-prefrontal synchrony and spatial working memory. J Neurosci 36:8372–8389
Harland BC, Collings DA, Mcnaughton N, Abraham WC, Dalrymple-Alford JC (2014) Anterior thalamic lesions reduce spine density in both hippocampal CA1 and retrosplenial cortex, but enrichment rescues CA1 spines only. Hippocampus 24:1232–1247
Hembrook JR, Onos KD, Mair RG (2012) Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus 22:853–860
Hongpaisan J, Alkon DL (2007) A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. Proc Natl Acad Sci USA 104:19571–19576
Hoover WB, Vertes RP (2012) Collateral projections from nucleus reuniens of thalamus to hippocampus and medial prefrontal cortex in the rat: a single and double retrograde fluorescent labeling study. Brain Struct Funct 217:191–209
Howell D (1992) Statistical methods for psychology. Duxbury Press, Belmont, p 338p
Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J (2010) Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci 33:121–129
Layfield DM, Patel M, Hallock H, Griffin AL (2015) Inactivation of the nucleus reuniens/rhomboid causes a delay-dependent impairment of spatial working memory. Neurobiol Learn Mem 125:163–167
Lesburgueres E, Gobbo OL, Alaux-Cantin S, Hambucken A, Trifilieff P, Bontempi B (2011) Early tagging of cortical networks is required for the formation of enduring associative memory. Science 331:924–928
Linley SB, Gallo MM, Vertes RP (2016) Lesions of the ventral midline thalamus produce deficits in reversal learning and attention on an odor texture set shifting task. Brain Res 1649:110–122
Lopez J, Herbeaux K, Cosquer B, Engeln M, Muller C, Lazarus C, Kelche C, Bontempi B, Cassel J-C, de Vasconcelos AP (2012) Context-dependent modulation of hippocampal and cortical recruitment during remote spatial memory retrieval. Hippocampus 22:827–841
Loureiro M, Cholvin T, Lopez J, Merienne N, Latreche A, Cosquer B, Geiger K, Kelche C, Cassel JC, Pereira de Vasconcelos A (2012) The ventral midline thalamus (reuniens and rhomboid nuclei) contributes to the persistence of spatial memory in rats. J Neurosci 32(29):9947–9959
Mahmmoud RR, Sase S, Aher YD, Sase A, Gröger M, Mokhtar M, Höger H, Lubec G (2015) Spatial and working memory is linked to spine density and mushroom spines. PloS One 10(10):e0139739
Maisson DJ, Gemzik ZM, Griffin AL (2018) Optogenetic suppression of the nucleus reuniens selectively impairs encoding during spatial working memory. Neurobiol Learn Memomry 155:78–85
Moser MB, Trommald M, Andersen P (1994) An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA 91:12673–12675
O’Malley A, O’Connell C, Murphy KJ, Regan CM (2000) Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience 99:229–232
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press/Elsevier, Amsterdam, Boston
Pereira AC, Lambert HK, Grossman YS, Dumitriu D, Waldman R, Jannetty SK, Calakos K, Janssen WG, McEwen BS, Morrison JH (2014) Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc Natl Acad Sci USA 111:18733–18738
Restivo L, Vetere G, Bontempi B, Ammassari-Teule M (2009) The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci 29:8206–8214
Roy A, Svensson FP, Mazeh A, Kocsis B (2017) Prefrontal-hippocampal coupling by theta rhythm and by 2–5 Hz oscillation in the delta band: the role of the nucleus reuniens of the thalamus. Brain Structe Funct 222:2819–2830
Ryan TJ, Roy DS, Pignatelli M, Arons A, Tonegawa S (2015) Engram cells retain memory under retrograde amnesia. Science 348(6238):1007–1013
Sanders J, Cowansage K, Baumgärtel K, Mayford K (2012) Elimination of dendritic spines with long-term memory is specific to active circuits. J Neurosci 32(36):12570–12578
Squire LR, Genzel L, Wixted JT, Morris RG (2015) Memory Consolidation. Cold Spring Harbor Perspect Biol 7:a021766
Thielen JW, Takashima A, Rutters F, Tendolkar I, Fernández G (2015) Transient relay function of midline thalamic nuclei during long-term memory consolidation in humans. Learn Memory 22:527–531
Thierry AM, Gioanni Y, Dégénétais E, Glowinski J (2000) Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus 10:411–419
Varela C, Kumar S, Yang JY, Wilson MA (2014) Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct 219:911–929
Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the Rat. Synapse 51:32–58
Vetere G, Restivo L, Cole CJ, Ross PJ, Ammassari-Teule M, Josselyn SA, Frankland PW (2011) Spine growth in the anterior cingulate cortex is necessary for the consolidation of contextual fear memory. Proc Natl Acad Sci USA 108:8456–8460
West MJ (2013) Getting started in stereology. Cold Spring Harbor Protoc 2013:287–297
West MJ, Slomianka L, Gundersen HJG (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hiuppocampus using the optical fractionator. Anat Rec 231:482–497
Xu W, Südhof TC (2013) A neural circuit for memory specificity and generalization. Science 339:1290–1295
Xu B, Sun A, He Y, Qian F, Liu L, Chen Y, Luo H (2017) Running-induced memory enhancement correlates with the preservation of thin spines in the hippocampal area CA1 of old C57BL/6 mice. Neurobiol Aging 52:106–116
Acknowledgements
This work was supported by the Agence Nationale de la Recherche (ANR grant 14-CE13-0029-01), the University of Strasbourg, the CNRS, INSERM, and by the Région Alsace (a territory now belonging to Région Grand Est) which contributed (50%) to the PhD fellowship allocated to M.M.K. (the supplement was provided by the ANR, same grant as above), as well as by the French government which provided a PhD fellowship to T.C. The authors acknowledge Olivier Bildstein, Daniel Egesi and George Edomwony for their precious contribution to animal care, as well as Mathilde Köhler, Manon Gerum, and Martin Deligny for providing appreciable technical help. We also thank Ms Delphine Cochand for her proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest. The study conformed to the rules of the European Community council directive of 22 September 2010 (2010-63) and of the French Department of Agriculture. All approaches have been validated by a local ethical committee (CREMEAS - authorization n° AL/32/39/02/13). The ANR grant did not depend on any particular clause which might have influenced data collection, analysis, interpretation and reporting.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Klein, M.M., Cholvin, T., Cosquer, B. et al. Ventral midline thalamus lesion prevents persistence of new (learning-triggered) hippocampal spines, delayed neocortical spinogenesis, and spatial memory durability. Brain Struct Funct 224, 1659–1676 (2019). https://doi.org/10.1007/s00429-019-01865-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-019-01865-1