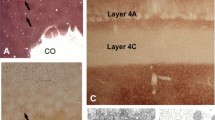
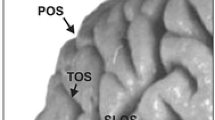
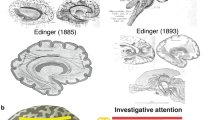
Abstract
Throughout history, researchers who examine the structure and function of the brain debate one another about how cortical areas are defined, as well as how these areas should be named. Different pieces of empirical evidence are used to define brain areas and it is important to preserve the accurate history of this evidence and the timeline of studies that lead to areal definitions that are either still used today or have been modified. As such, this paper traces the early history of a brain area located at the junction between the occipital and temporal lobes of the macaque known as TEO. This historical analysis leads to four main findings. First, even though Bonin and Bailey are credited with the definition of area TEO in 1947, they did not have the cytoarchitectonic evidence to support the distinction of TEO from adjacent areas. Second, the first evidence definitively separating area TEO from TE was actually based on connectivity as identified with strychnine neuronography by Petr et al. in 1949. Third, causal evidence from ablation studies conducted by Iwai and Mishkin (Experimental Neurology 25(4):585–594, 1969) supported this distinction by showing that TEO and TE were functionally distinct from one another. Fourth, researchers in the 1970s began referring to TEO as posterior inferotemporal (PIT) and TE as anterior inferotemporal (AIT), which is an important historical clarification as the PIT/AIT nomenclature is presently attributed to studies conducted more than a decade later. Altogether, this paper aims to preserve the historical origin of area TEO, as well as the empirical evidence that was used to originally differentiate this cortical expanse from surrounding areas.






Similar content being viewed by others
Notes
During the time periods discussed in the present paper, pioneering brain research was being conducted with different types of methods. However, the focus of the present paper is on the studies of cortical ablation and strychnine neuronography as they are critical historically for the original distinction among areas TE, TEO, and OA. Pioneering neurophysiology studies of TE and TEO (Gross et al. 1969, 1972; Boussaoud et al. 1991; and many others) were also being conducted during the time periods discussed in the present paper, but discussion of these studies is beyond the scope of the present review. I should also clarify that particular attention is placed on studies conducted by Iwai et al. as many of them are conference proceedings that remain in the stacks of libraries. As such, they are largely excluded from the modern literature, but are integral for the historical origin of TEO.
If such a description does exist in the literature and I have missed it, I am hopeful that a reader would contact me or write a letter to the journal (or both) to assure that the history of TEO is preserved.
Initially, it was unclear as to why Bonin and Bailey cited Economo (1929) consistently throughout their monograph instead of the original Economo and Koskinas (1925) atlas that was published four years prior. But, in a paper published by Peden and Bonin (1947) in the same year as the Bonin and Bailey monograph, a footnote revealed that the reason was because of availability. Peden and Bonin write: “We prefer to cite Economo’s English summary since it is more readily available than the costly cytoarchitectural atlas published with Koskinas in 1925.” Peden and Bonin (1947), pg. 40.
Consistent with this idea, Triarhou (2007b) refers to area PHP as the “Basal (temporooccipital) parietal area at parietal entrance,” area PHT as the “Basal (temporooccipital) parietal area at temporal entrance,” and PHO as the “Basal (temporooccipital) parietal area at occipital entrance” (Triarhou 2007b, Table 2, pg. 208).
Interestingly, while Bonin and Bailey did not include TEO in the frontispiece of their 1947 monograph of the macaque brain, they did include area PH on the frontispiece of their 1950 monograph (with McCulloch) of the chimpanzee brain.
Cowey and Gross (1970) included a footnote in their paper with similar concerns. They write: “The terminology for the subdivisions of the non-striate visual areas of the occipital and temporal lobes of the monkey is still rather confusing. This is hardly surprising, for the subdivision of these areas on cytoarchitectonic grounds by different authorities is contradictory and the study of the properties of single units in these areas has only begun. Although the recent demonstrations by Zeki (1969) and Cragg and Ainsworth (1969) that lateral striate cortex has two topographic and a third non-topographic projection onto prestriate cortex is a major step forward, the exact boundaries of these projections and their detailed relations to the various cytoarchitectonic subdivisions and subdivisions based on electrophysiological data are not yet entirely clear. Since the terminology used in behavioral studies of lesions of the non-striate visual areas is also inconsistent, it may be helpful to explain the terminology we have used in this report. We have called our posterior lesions “foveal prestriate lesions” because they include the entire area of prestriate cortex to which foveal striate cortex selectively projects (Zeki 1969; Cragg and Ainsworth 1969). Simply to call them prestriate cortex lesions or posterior inferotemporal cortex lesions is misleading because they include far less than the former and far more than the latter. Our inferotemporal lesions correspond closely to Von Bonin and Bailey’s “Area TE”. However, numerous publications on the behavioral effects of removing “inferotemporal” cortex illustrate lesions which often extend posterior to “Area TE” as far as the inferior occipital sulcus and thus may include part of foveal prestriate cortex. We therefore wish to stress that our inferotemporal lesions are restricted to area TE and may not be comparable to some “inferotemporal” lesions in other studies.” Cowey and Gross (1970), Pgs. 128–129.
Though Zeki (1996) cites Felleman and Van Essen (1991) for the definition of PIT, Van Essen and colleagues re-proposed the definition and name of PITd and PITv in a different paper the year prior (Van Essen et al. 1990) in which they write: “The fact that there are two pairs of topographically organized foci suggests that they form two distinct areas, which we have termed PITd and PITv (dorsal and ventral subdivisions of the posterior inferotempo-ral area).” Van Essen et al. (1990, pg. 688).
References
Afraz A, Boyden EA, DiCarlo JJ (2015) Optogenetic and pharmacological suppression of spatial clusters of face neurons reveal their causal role in face gender discrimination. PNAS 112(21):6730–6735
Amunts K, Zilles K (2015) Architectonic mapping of the human brain beyond Brodmann. Neuron 88(6):1086–1107
Arcaro MJ, Livingstone MS (2017) Retinotopic organization of scene areas in macaque inferior temporal cortex. J Neurosci 37(31):7373–7389
Bailey P, Bonin GV, Garol HW, McCulloch WS (1943) Long association fibers in cerebral hemispheres of monkey and chimpanzee. J Neurophysiol 6(2):129–134
Bailey P, Bonin GV, McCulloch WS (1950) The isocortex of the chimpanzee. University of Illinois Press, Urbana
Bender DB (1973) Visual sensitivity following inferotemporal and foveal prestriate lesions in the rhesus monkey. J Comp Physiol Psychol 84:613–621
Bender DB, Gross CG (1981) Backward masking in monkeys after foveal prestriate and inferior temporal cortex lesions. Physiol Psychol 9(3):257–259
Bertini G, Buffalo EA, De Weerd P, Desimone R, Ungerleider LG (2004) Visual responses to targets and distractors by inferior temporal neurons after lesions of extrastriate areas V4 and TEO. Neuroreport 15(10):1611–1615
Bolster B, Crowne DP (1979) Effects of anterior and posterior inferotemporal lesions on discrimination reversal in the monkey. Neuropsychologia 17:11–20
Bonin GV, Bailey P (1947) The neocortex of Macaca mulatta. University of Illinois Press, Urbana
Boussaoud D, Desimone R, Ungerleider LG (1991) Visual topography of area TEO in the macaque. J Comp Neurol 306(4): 554–75
Brewer AA, Press WA, Logothetis NK, Wandell BA (2002) Visual areas in macaque cortex measured using functional magnetic resonance imaging. J Neurosci 22(23):10416–10426
Brodmann K (1907) Beitraege zur histologischen Lokalisation der Grosshirnrinde. Vite Mitteilung: Die Cortexgliederung des Menschen. J Psychol Neurol 10:231–246
Buffalo EA, Bertini G, Ungerleider LG, Desimone R (2005) Impaired filtering of distractor stimuli by TE neurons following V4 and TEO lesions in macaques. Cereb Cortex 15(2):141–151
Christensen CA, Pribram KH (1977) The visual discrimination performance of monkeys with foveal prestriate and inferotemporal lesions. Physiol Behavior 18:403–407
Christensen C, Pribram K (1979) The effect of inferotemporal or foveal prestriate ablation on serial reversal learning in monkeys. Neuropsychologia 17(1):1–10
Cragg BG, Ainsworth A (1969) The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res 9:737–747
Cowey A, Gross CG (1970) Effects of foveal prestriate and inferotemporal lesions on visual discrimination by rhesus monkeys. Exp Brain Res 11:128–144
De Weerd P, Desimone R, Ungerleider LG (2003ab) Impairments in spatial generalization of visual skills after V4 and TEO lesions in macaques (Macaca mulatta). Behav Neurosci 117(6):1441–1447
De Weerd P, Desimone R, Ungerleider LG (2003ba) Generalized deficits in visual selective attention after V4 and TEO lesions in macaques. Eur J Neurosci 18(6):167–191
Dean P (1976) Effects of inferotemporal lesions on the behavior of monkeys. Psychol Bull 83(1):41–71
Desimone R, Fleming J, Gross CG (1980) Prestriate afferents to inferior temporal cortex: an HRP study. Brain Res 184:41–55
Economo C (1929) The cytoarchitecture of the human cerebral cortex. Oxford University Press, New York
Economo C, Koskinas GN (1925) Cytoarchitektonik der Grosshirnrinde des erwachsenen Menschen. Springer, Berlin
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47
Fenstemaker SB (1986) The organization and connections of visual cortical area TEO in the macaque. Ph.D. thesis, Princeton University. New Jersey
Fisher C, Freiwald WA (2015) Whole-agent selectivity within the macaque face-processing system. PNAS 112(47):14717–14722
Fize D, Vanduffel W, Nelissen K, Denys K, Chef d’Hotel C, Faugeras O, Orban GA (2003) The retinotopic organization of primate dorsal V4 and surrounding areas: a functional magnetic resonance imaging study in awake monkeys. J Neurosci 23(19):7395–7406
Grill-Spector K, Weiner KS (2014) The functional architecture of the ventral temporal cortex and its role in categorization. Nat Rev Neurosci 15:536–548
Gross CG (1973) Visual functions of inferotemporal cortex. In: Jung R (ed) Handbook of sensory physiology, vol II, Part 3B. Springer, Berlin, pp 451–482
Gross CG (1994) How inferior temporal cortex became a visual area. Cereb Cortex 5:455–469
Gross CG, Bender DB, Rocha-Miranda CE (1969) Visual receptive fields of neurons in inferotemporal cortex of the monkey. Science 166(3910):1303–1306
Gross CG, Rocha-Miranda CE, Bender DB (1972) Visual properties of neurons in inferotemporal cortex of the Macaque. J Neurophysiol 35(1):96–111
Iversen SD (1973a) Visual discrimination deficits associated with posterior inferotemporal lesions in the monkey. Brain Res 62:89–101
Iversen SD (1973b) Brain lesions and memory in animals. In: Deutsch AJ (eds) The physiological basis of memory. Academic Press, New York, pp 305–364
Iversen SD, Humphrey NK (1971) Ventral temporal lobe lesions and visual oddity performance. Brain Res 30:253–263
Iwai E (1971) Experimental visual agnosia. Advanc Neurol Sci 71–86 (in Japanese)
Iwai E (1978) The visual learning area in the inferotemporal cortex of monkeys. In: Ito M, Kubota K, Tsukahara N, Yagi K (eds) Integrative control functions of the brain, vol I. Elsevier, New York, pp 419–427
Iwai E (1980) Visual mechanisms in the temporal and prestriate association cortices of the monkey. In: Adam G, Meszaros I, Banyai EI (eds) Brain and behaviour. Pergamon Press, New York, pp 279–286
Iwai E (1982) A model regarding system of information processing in visual goal-directed behavior of macaque monkeys. In: Saito S, McGaugh JL (eds) Learning and memory. Excerpta Medica, Amsterdam, pp 39–58
Iwai E, Mishkin M (1969) Further evidence on the locus of the visual area in the temporal lobe of the monkey. Exp Neurol 25(4):585–594
Iwai E, Yukie M (1987) Amygdalofugal and amygdalopetal connections with modality-specific visual cortical areas in macaques (Macaca fuscata, M. mulatta, and M. fascicularis). J Comp Neurol 261:362–387
Iwai E, Yukie M, Watanabe J, Kazuo H, Suyama H, Ishikawa S (1990) A role of amygdala in visual perception and cognition in macaque monkeys (Macaca fuscata and Macaca mulatta). Tohoku J Exp Med 161:95–120
Janssens T, Zhu Q, Popivanov ID, Vanduffel W (2014) Probabilistic and single-subject retinotopic maps reveal the topographic organization of face patches in the macaque cortex. J Neurosci 34(31):10156–10167
Keating EG (1975) Effects of prestriate and striate lesions on the monkey's ability to locate and discriminate visual forms. Exp Neurol 47:16–25
Kikuchi R, Iwai E (1980) The locus of the posterior subdivision of the inferotemporal visual learning area in the monkey. Brain Res 198:347–360
Kolster H, Janssens T, Orban GA, Vanduffel W (2014) The retinotopic organization of macaque occipitotemporal cortex anterior to V4 and caudoventral to the middle temporal (MT) cluster. J Neurosci 34(31):10168–10191
Kornblith S, Cheng X, Ohayon S, Tsao DY (2013) A network for scene processing in the macaque temporal lobe. Neuron 79(4):766–781
Lafer-Sousa R, Conway BR (2013) Parallel, multi-stage processing of colors, faces, and shapes in macaque inferior temporal cortex. Nat Neurosci 16(12):1870–1878
Manning FJ (1971) Punishment for errors and visual discrimination learning by monkeys with inferotemporal lesions. J Comp Physiol Psychol 75:146–152
Manning FJ (1972) Serial reversal learning by monkeys with inferotemporal and foveal prestriate lesions. Physiol Behav 8:177–181
McCulloch WS (1944) The functional organization of the cerebral cortex. Physiol Rev 24(3):390–407
Mishkin M (1972) Cortical visual areas and their interactions. In: Karczmer AG, Eccles JC (eds) Brain and human behavior. Springer, Berlin, pp 187–208
Mishkin M, Ungerleider LG, Macko KA (1983) Object vision and spatial vision: two cortical pathways. Trends Neurosci 6:414–417
Moeller S, Freiwald WA, Tsao DY (2008) Patches with links: a unified system for processing faces in the macaque temporal lobe. Science 320(5881):1355–1359
Moeller S, Crapse T, Chang L, Tsao DY (2017) The effect of face patch microstimulation of faces and objects. Nat Neurosci 20(5):743–752
Peden JK, Bonin G von (1947) The neocortex of Hapale. J Comp Neurol 86:37–63
Petr R, Holden LB, Jirout J (1949) The efferent intercortical connections of teh superficial corten of the temporal lobe (macaca mulatta)*. J Neuropathol Exp Neurol 8(1):100–103
Pinsk MA, Arcaro M, Weiner KS, Kalkus JF, Inati SJ, Gross CG, Kastner S (2009) Neural representations of faces and body parts in macaque and human cortex: a comparative fMRI study. J Neurophysiol 101(5):2581–2600
Sadagopan S, Zarco W, Freiwald WA (2017) A causal relationship between face-patch activity and face-detection behavior. eLife. https://doi.org/10.7554/eLife.18558
Saghal A, Iversen SD (1978) Categorization and retrieval after selective inferotemporal lesions in monkeys. Brain Res 146:341–350
Takemura H, Rokem A, Winawer J, Yeatman JD, Wandell BA, Pestilli F (2016) A major human white-matter pathway between dorsal and ventral visual cortex. Cereb Cortex 26(5):2205–2214
Takemura H, Pestilli F, Weiner KS, Keliris GA, Landi SM, Sliwa J, Ye FQ, Barnett MA, Leopold DA, Freiwald WA, Logothetis NK, Wandell BA (2017) Occipital white matter tracts in human and macaque. Cereb Cortex 27(6):3346–3359
Tootell RB, Tsao D, Vanduffel W (2003) Neuroimaging weighs in: humans meet macaques in “primate” visual cortex. J Neurosci 23:3981–3989
Triarhou LC (2006) The signalling contributions of Constantin von Economo to basic, clinical and evolutionary neuroscience. Brain Res Bull 69:223–243
Triarhou LC (2007a) The Economo–Koskinas atlas revisited: cytoarchitectonics and functional context. Stereotact Funct Neurosurg 85(5):195–203
Triarhou LC (2007b) A proposed number system for the 107 cortical areas of Economo and Koskinas, and Brodmann area correlations. Stereotact Funct Neurosurg 85(5):204–215
Triarhou LC (2005) Georg N Koskinas (1885–1975) and his scientific contributions to the normal and pathological anatomy of the human brain. Brain Res Bull 68:121–139
Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB (2003) Faces and objects in macaque cerebral cortex. Nat Neurosci 6(9):989–995
Tsao DY, Freiwald WA, Tootell RB, Livingstone MS (2006) A cortical region consisting entirely of face-selective cells. Science 311(5761):670–674
Umitsu Y, Iwai E (1980) The posterior inferotemporal cortex as an anatomically distinguishable area from adjacent cortical areas of the anterior inferotemporal cortex and the prestriate cortex. In: Ito M, Kubota K, Tsukahara N, Yagi K (eds) Integrative control functions of the brain, vol III. Elsevier, New York, pp 384–386
Ungerleider LG, Mishkin M (1982) Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW (eds) Analysis of visual behavior. MIT Press, Cambridge, pp 549–586
Van Essen DC (2003) Organization of visual areas in macaque and human cerebral cortex. In: Chalupa LM, Werner JS (eds) The visual neurosciences. Bradford Books, Boston, pp. 507–521
Van Essen DC, Felleman DJ, DeYoe EA, Olavarria J, Knierim J (1990) Modular and hierarchical organization of extrastriate visual cortex in the macaque monkey. Cold Spring Harb Symp Quant Biol 55:679–696
Van Essen DC, Glasser MF, Dierker DL, Harwell J (2012aa) Cortical parcellations of the macaque monkey analyzed on surface-based atlases. Cereb Cortex 22(10):2227–2240
Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T (2012b) Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex 22(10):2241–2262
Wade A, Augath M, Logothetis N, Wandell BA (2008) fMRI measurements of color in macaque and human. J Vis 8(10):1–19
Weiner KS, Barnett MA, Lorenz S, Caspers J, Stigliani A, Amunts K, Zilles K, Fischl B, Grill-Spector K (2017) The cytoarchitecture of domain-specific regions in human high-level visual cortex. Cereb Cortex 27(1):146–161
Wilson M, Kaufman HM, Zieler RE, Lieb JP (1972) Visual identification and memory in monkeys with circumscribed inferotemporal lesions. J Comp Physiol Psychol 78(2):173–183
Yue X, Pourladian IS, Tootell RB, Ungerleider LG (2014) Curvature-processing network in macaque visual cortex. PNAS 111(33):E3467–E3475
Zeki SM (1969) Representation of central visual fields in prestriate cortex of monkey. Brain Res. 14:271–291
Zeki SM (1971) Cortical projections from two prestriate areas. Brain Res 34:19–35
Zeki SM (1977) Colour coding in the superior temporal sulcus of the rhesus monkey visual cortex. Proc R Soc B Biol Sci 197:195–223
Zeki S (1996) Are areas TEO and PIT of monkey visual cortex wholly distinct from the fourth visual complex (V4 complex)? Proc R Soc B Biol Sci 263(1376):1539–1544
Acknowledgements
I thank Mona Rosenke for comments on a previous version of this manuscript.
Funding
This research was funded by NEI Grant 1 RO1 EY 02391501A1 and start-up funds provided by UC Berkeley.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing financial interests associated with this article.
Research involving human participants and/or animals, ethical approval, and informed consent
The content of this paper is a historical review and the author did not conduct any experiments on humans or animals.
Rights and permissions
About this article
Cite this article
Weiner, K.S. Area TEO and “Area ?”: cytoarchitectonic confusion corrected by connectivity and cortical ablation. Brain Struct Funct 223, 3515–3529 (2018). https://doi.org/10.1007/s00429-018-1714-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1714-1