Abstract
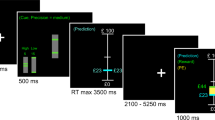
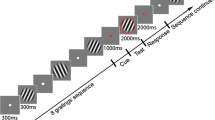
Parkinson’s disease (PD), which is caused by degeneration of dopaminergic neurons in the midbrain, results in a heterogeneous clinical picture including cognitive decline. Since the phasic signal of dopamine neurons is proposed to guide learning by signifying mismatches between subjects’ expectations and external events, we here investigated whether akinetic-rigid PD patients without mild cognitive impairment exhibit difficulties in dealing with either relevant (requiring flexibility) or irrelevant (requiring stability) prediction errors. Following our previous study on flexibility and stability in prediction (Trempler et al. J Cogn Neurosci 29(2):298–309, 2017), we then assessed whether deficits would correspond with specific structural alterations in dopaminergic regions as well as in inferior frontal cortex, medial prefrontal cortex, and the hippocampus. Twenty-one healthy controls and twenty-one akinetic-rigid PD patients on and off medication performed a task which required to serially predict upcoming items. Switches between predictable sequences had to be indicated via button press, whereas sequence omissions had to be ignored. Independent of the disease, midbrain volume was related to a general response bias to unexpected events, whereas right putamen volume correlated with the ability to discriminate between relevant and irrelevant prediction errors. However, patients compared with healthy participants showed deficits in stabilisation against irrelevant prediction errors, associated with thickness of right inferior frontal gyrus and left medial prefrontal cortex. Flexible updating due to relevant prediction errors was also affected in patients compared with controls and associated with right hippocampus volume. Dopaminergic medication influenced behavioural performance across, but not within the patients. Our exploratory study warrants further research on deficient prediction error processing and its structural correlates as a core of cognitive symptoms occurring already in early stages of the disease.





Similar content being viewed by others
References
Alexander WH, Brown JW (2011) Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci 14(10):1338–1344
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9(1):357–381
Aron AR (2007) The neural basis of inhibition in cognitive control. Neurosci 13(3):214–228
Baddeley AD (1986) Working memory. Oxford University Press, Oxford
Badre D (2012) Opening the gate to working memory. Proceedings of the National Academy of Sciences, USA, 109, 19878-79
Baker JE (1987) Reducing Bias and Inefficiency in the Selection Algorithm. Proceedings of the Second International Conference on Genetic Algorithms and their Application, 14–21
Bilder RM, Volavka J, Lachman HM, Grace AA (2004) The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29:1943–1961
Biundo R, Calabrese M, Weis L, Facchini S, Ricchieri G, Gallo P, Antonini A (2013). Anatomical correlates of cognitive functions in early Parkinson’s disease patients. PLoS One, 8(5):e64222
Biundo R, Weis L, Antonini A (2016) Cognitive decline in Parkinson’s disease: the complex picture. NPJ Parkinson’s Dis 2:16018
Braak H, Del Tredici K, Rüb U, De Vos RA, Steur ENJ, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Cerasa A, Morelli M, Augimeri A, Salsone M, Novellino F, Gioia MC, Arabia G, Quattrone A (2013) Prefrontal thickening in PD with levodopa-induced dyskinesias: new evidence from cortical thickness measurement. Parkinsonism Relat Disord 19(1):123–125
Chase TN (1990) Motor response complications with chronic levodopa therapy. Adv Neurol 53:377–381
Chatham CH, Badre D (2015) Multiple gates on working memory. Curr Opin Behav Sci 1:23–31
Chaudhuri KR, Schapira AH (2009). Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol, 8(5), 464 – 74
Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD (2011) Associative retrieval processes in the human medial temporal lobe: hippocampal retrieval success and CA1 mismatch detection. Learn Memory 18(8):523–528
Cohen JD, Braver TS, Brown JW (2002) Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol 12(2):223–229
Cools R (2006) Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev 30(1):1–23
Cools R (2011) Dopaminergic control of the striatum for high-level cognition. Curr Opin Neurobiol 21(3):402–407
Cools R, D’Esposito M (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiat 69:e113-25
Cools R, Barker RA, Sahakian BJ, Robbins TW (2003) L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia 41(11):1431–1441
Cools R, Sheridan M, Jacobs E, D’Esposito M (2007) Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci 27:5506–5514
Cools R, Miyakawa A, Sheridan M, D’Esposito M (2009). Enhanced frontal function in Parkinson’s disease. Brain awp301
D’Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD (2012). Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proceedings of the National Academy of Sciences, USA, 109, 19900-9
D’Esposito M, Postle BR (2015) The cognitive neuroscience of working memory. Annu Rev Psychol 66:115–142
Dale AM, Fischl B, Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179 – 94
Delaville C, De Deurwaerdère P, Benazzouz A (2011). Noradrenaline and Parkinson’s disease. Front Syst Neurosci, 5
Depue BE, Burgess GC, Bidwell LC, Willcutt EG, Banich MT (2010) Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Res Neuroimaging 182(3):231–237
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31(3):968–980
di Pellegrino G, Ciaramelli E, Làdavas E (2007) The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci 19(2):275–286
Durstewitz D, Seamans JK (2008) The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiat 64:739–749
Durstewitz D, Seamans JK, Sejnowski TJ (2000) Neurocomputational models of working memory. Nat Neurosci 3:1184–1191
Fahn S, Elton RL (1987). Unified rating scale for Parkinson’s disease. Recent developments in Parkinson’s Disease, 153 – 63
Fegen D, Buchsbaum BR, D’Esposito M (2015) The effect of rehearsal rate and memory load on verbal working memory. NeuroImage 105:120–131
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050-5
Frank MJ, Loughry B, O’Reilly RC (2001) Interactions between the frontal cortex and basal ganglia in working memory: a computational model. Cognit Affective Behav Neurosci 1:137–160
Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ (2011) Disability, atrophy and cortical reorganization following spinal cord injury. Brain 134:1610–1622
Fröber K, Dreisbach G (2017) Keep flexible–Keep switching! The influence of forced task switching on voluntary task switching. Cognition 162:48–53
Galea JM, Bestmann S, Beigi M, Jahanshahi M, Rothwell JC (2012). Action reprogramming in Parkinson’s disease: response to prediction error is modulated by levels of dopamine. J Neurosci 32(2):542–550
Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9(1):13–24
Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G, Kirik D (2014). Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain, 137(9):2493–2508
Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010) The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage 50(3):1313–1319
Hautzinger M, Keller F, Kühner C (2006) Beck depressions-inventar (BDI-II). Harcourt Test Services, Frankfurt
Henry RG, Shieh M, Amirbekian B, Chung S, Okuda DT, Pelletier D (2009) Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J Neurol Sci 282:61–66
Hiebert NM, Vo A, Hampshire A, Owen AM, Seergobin KN, MacDonald PA (2014) Striatum in stimulus–response learning via feedback and in decision making. NeuroImage 101:448–457
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Houk JC, Wise SP (1995) Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cerebral Cortex 5(2):95–110
Howard CD, Li H, Geddes CE, Jin X (2017) Dynamic nigrostriatal dopamine biases action selection. Neuron 93(6):1436–1450
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Humphries MD, Stewart RD, Gurney KN (2006) A physiologically plausible model of action selection and oscillatory activity in the basal ganglia. J Neurosci 26(50):12921–12942
Iglesias JE, Van Leemput K, Bhatt P, Casillas C, Dutt S, Schuff N, Truran-Sacrey D, Boxer A, Fischl B (2015) Bayesian segmentation of brainstem structures in MRI. NeuroImage 113:184–195
Jueptner M, Weiller C (1998) A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain J Neurol 121(8):1437–1449
Kalbe E, Calabrese P, Kohn N, Hilker R, Riedel O, Wittchen HU, Dodel R, Otto J, Ebersbach G, Kessler J (2008) Screening for cognitive deficits in Parkinson’s disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Rel Disord 14(2):93–101
Kehagia AA, Barker RA, Robbins TW (2010) Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol 9(12):1200–1213.
Kehagia AA, Barker RA, Robbins TW (2012) Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegenerative Dis 11(2):79–92
Lavie N (2005) Distracted and confused?: Selective attention under load. Trends Cognit Sci 9:75–82
Lo CC, Xiao-Jing W (2006) Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci 9(7):956
Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, Del Campo N, … Bullmore E (2007) Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain 130(12):3223–3236
Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202
Muslimović D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65(8):1239–1245
Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–74
Price CC, Tanner J, Nguyen PT, Schwab NA, Mitchell S, Slonena E, Brumback B, Okun MS, Mareci TH, Bowers D (2016). Gray and white matter contributions to cognitive frontostriatal deficits in non-demented Parkinson’s disease. PloS One, 11(1):e0147332
Redgrave P, Gurney K (2006). The short-latency dopamine signal: a role in discovering novel actions?. Nat Rev Neurosci 7(12):967–75
Rektorova I, Biundo R, Marecek R, Weis L, Aarsland D, Antonini A (2014). Grey matter changes in cognitively impaired Parkinson’s disease patients. PloS One, 9(1):e85595
Ross RS, Brown TI, Stern CE (2009) The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus 19(9):790–799
Schiffer AM, Ahlheim C, Wurm MF, Schubotz RI (2012). Surprised at all the entropy: hippocampal, caudate and midbrain contributions to learning from prediction errors. PloS One, 7(5), e36445
Schiffer AM, Waszak F, Yeung N (2015) The role of prediction and outcomes in adaptive cognitive control. J Physiol Paris 109(1):38–52
Schlichting ML, Preston AR (2015) Memory integration: neural mechanisms and implications for behavior. Curr Opin Behav Sci 1:1–8
Schonberg T, O‘Doherty JP, Joel D, Inzelberg R, Segev Y, Daw ND (2010). Selective impairment of prediction error signaling in human dorsolateral but not ventral striatum in Parkinson’s disease patients: evidence from a model-based fMRI study. NeuroImage, 49(1), 772 – 81
Schönberger AR, Barbe MT, Hagelweide K, Kühn AB, Fink GR, Schubotz RI (2013) Joint principles of motor and cognitive dysfunction in Parkinson’s disease. Neuropsychologia 51(8):1417–1425
Schott BH, Niehaus L, Wittmann BC, Schütze H, Seidenbecher CI, Heinze HJ, Düzel E (2007) Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain 130(9):2412–2424
Schubotz RI (2007) Prediction of external events with our motor system: towards a new framework. Trends Cognitive Sci 11(5):211–218
Schultz W, Dickinson A (2000) Neuronal coding of prediction errors. Annu Rev Neurosci 23:473–500
Shergill SS, Brammer MJ, Fukuda R, Bullmore E, Amaro E Jr, Murray RM, McGuire PK (2002) Modulation of activity in temporal cortex during generation of inner speech. Hum Brain Mapp 16:219–227
Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, Ota T, Asahina M, Fukushi K, Kuwabara S, Hattori T, Suhara T, Irie T (2009) Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 73:273–278
Snodgrass JG, Corwin J (1988) Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 117(1):34
Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ (2010) Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci 30(42):14205–14212
Tessitore A, Santangelo G, De Micco R, Vitale C, Giordano A, Raimo S, Corbo D, Amboni M, Barone P, Tedeschi G (2016) Cortical thickness changes in patients with Parkinson’s disease and impulse control disorders. Parkinsonism Rel Disord 24:119–125
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement Disord 25(15):2649–2653
Trempler I, Schiffer AM, El-Sourani N, Ahlheim C, Fink GR, Schubotz RI (2017) Frontostriatal contribution to the interplay of flexibility and stability in serial prediction. J Cogn Neurosci 29(2):298–309
Vaillancourt DE, Schonfeld D, Kwak Y, Bohnen NI, Seidler R (2013) Dopamine overdose hypothesis: evidence and clinical implications. Movement Disord 28(14):1920–1929
Vazey EM, Aston-Jones G (2012). The emerging role of norepinephrine in cognitive dysfunctions of Parkinson’s disease. Front Behav Neurosci, 6
Villain N, Fouquet M, Baron JC, Mézenge F, Landeau B, de La Sayette V, Viader F, Eustache F, Desgranges B, Chételat G (2010) Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain 133(11):3301–3314
Zimmermann P, Fimm B (1993) Testbatterie zur Aufmerksamkeitsprüfung (TAP). Psytest, Freiburg
Acknowledgements
We sincerely thank the participants involved in the current investigation and Paul Reker for the UPDRS III video rating. We also acknowledge Klara Hagelweide for proof-reading and commenting on earlier versions of the manuscript. We are grateful to Anna Kuhns, Pascasie Leonie Dombert, Alexander Geiger, and Eileen Oberwelland for their assistance in data acquisition. We thank the German Research Foundation (Clinical Research Group KFO219 “Basal-Ganglia-Cortex-Loops: Mechanisms of Pathological Interactions and Therapeutic Modulation”, SCHU 1439/5 − 2) for financially supporting the project. Finally, we are thankful for the insightful and productive comments by our reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trempler, I., Binder, E., El-Sourani, N. et al. Association of grey matter changes with stability and flexibility of prediction in akinetic-rigid Parkinson’s disease. Brain Struct Funct 223, 2097–2111 (2018). https://doi.org/10.1007/s00429-018-1616-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1616-2