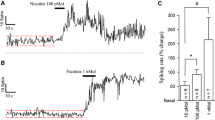
Abstract
Cholinergic neurons within the pedunculopontine tegmental nucleus have been implicated in a range of functions, including behavioral state control, attention, and modulation of midbrain and basal ganglia systems. Previous experiments with excitotoxic lesions have found persistent learning impairment and altered response to nicotine following lesion of the posterior component of the PPTg (pPPTg). These effects have been attributed to disrupted input to midbrain dopamine systems, particularly the ventral tegmental area. The pPPTg contains a dense collection of cholinergic neurons and also large numbers of glutamatergic and GABAergic neurons. Because these interdigitated populations of neurons are all susceptible to excitotoxins, the effects of such lesions cannot be attributed to one neuronal population. We wished to assess whether the learning impairments and altered responses to nicotine in excitotoxic PPTg-lesioned rats were due to loss of cholinergic neurons within the pPPTg. Selective depletion of cholinergic pPPTg neurons is achievable with the fusion toxin Dtx-UII, which targets UII receptors expressed only by cholinergic neurons in this region. Rats bearing bilateral lesions of cholinergic pPPTg neurons (>90 % ChAT+ neuronal loss) displayed no deficits in the learning or performance of fixed and variable ratio schedules of reinforcement for pellet reward. Separate rats with the same lesions had a normal locomotor response to nicotine and furthermore sensitized to repeated administration of nicotine at the same rate as sham controls. Previously seen changes in these behaviors following excitotoxic pPPTg lesions cannot be attributed solely to loss of cholinergic neurons. These findings indicate that non-cholinergic neurons within the pPPTg are responsible for the learning deficits and altered responses to nicotine seen after excitotoxic lesions. The functions of cholinergic neurons may be related to behavioral state control and attention rather than learning.











Similar content being viewed by others
References
Ainge JA, Keating GL, Latimer MP, Winn P (2006) The pedunculopontine tegmental nucleus and responding for sucrose reward. Behav Neurosci 120:563–570
Alderson HL, Latimer MP, Blaha CD, Phillips AG, Winn P (2004) An examination of d-amphetamine self-administration in pedunculopontine tegmental nucleus-lesioned rats. Neuroscience 125:349–358
Alderson HL, Latimer MP, Winn P (2006) Intravenous self-administration of nicotine is altered by lesions of the posterior, but not anterior, pedunculopontine tegmental nucleus. Eur J Neurosci 23:2169–2175
Alderson HL, Latimer MP, Winn P (2008) A functional dissociation of the anterior and posterior pedunculopontine tegmental nucleus: excitotoxic lesions have differential effects on locomotion and the response to nicotine. Brain Struct Funct 213:247–253
Bechara A, Vanderkooy D (1989) The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J Neurosci 9:3400–3409
Benwell ME, Balfour DJ (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105:849–856
Berdichevsky E, Riveros N, Sanchez-Armass S, Orrego F (1983) Kainate, N-methylaspartate and other excitatory amino acids increase calcium influx into rat brain cortex cells in vitro. Neurosci Lett 36:75–80
Wang HL, Chakraborti A, NG T, Yamaguchi T, Morales M (2010) Ventral tegmental input from the pedunculopontine and laterodorsal tegmental nuclei is dominated by glutamatergic and GABAergic, rather than cholinergic neurons. Program No. 366.4/FF8 2010. Neuroscience Meeting Planner. Society for Neuroscience, San Diego
Chen L, Lodge DJ (2013) The lateral mesopontine tegmentum regulates both tonic and phasic activity of VTA dopamine neurons. J Neurophysiol 110:2287–2294
Clark SD, Nothacker HP, Wang Z, Saito Y, Leslie FM, Civelli O (2001) The urotensin II receptor is expressed in the cholinergic mesopontine tegmentum of the rat. Brain Res 923:120–127
Clark SD, Alderson HL, Winn P, Latimer MP, Nothacker HP, Civelli O (2007) Fusion of diphtheria toxin and urotensin II produces a neurotoxin selective for cholinergic neurons in the rat mesopontine tegmentum. J Neurochem 102:112–120
Corbit LH, Balleine BW (2000) The role of the hippocampus in instrumental conditioning. J Neurosci 20:4233–4239
Cyr M, Parent MJ, Mechawar N, Rosa-Neto P, Soucy JP, Clark SD, Aghourian M, Bedard MA (2014) Deficit in sustained attention following selective cholinergic lesion of the pedunculopontine tegmental nucleus in rat, as measured with both post-mortem immunocytochemistry and in vivo PET imaging with [F]fluoroethoxybenzovesamicol. Behav Brain Res 278C:107–114
Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Bolam JP, Gerdjikov T, Mena-Segovia J (2014) A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem. J Neurosci 34:4509–4518
Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H (1991) Learning disturbances following excitotoxic lesion of cholinergic pedunculo-pontine nucleus in the rat. Brain Res 544:126–132
Deurveilher S, Hennevin E (2001) Lesions of the pedunculopontine tegmental nucleus reduce paradoxical sleep (PS) propensity: evidence from a short-term PS deprivation study in rats. Eur J Neurosci 13:1963–1976
Farquhar MJ, Latimer MP, Winn P (2012) Nicotine self-administered directly into the VTA by rats is weakly reinforcing but has strong reinforcement enhancing properties. Psychopharmacology (Berl) 220:43–54
Govind AP, Vezina P, Green WN (2009) Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol 78:756–765
Grillner P, Svensson TH (2000) Nicotine-induced excitation of midbrain dopamine neurons in vitro involves ionotropic glutamate receptor activation. Synapse 38:1–9
Hallanger AE, Wainer BH (1988) Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol 274:483–515
Inglis WL, Allen LF, Whitelaw RB, Latimer MP, Brace HM, Winn P (1994) An investigation into the role of the pedunculopontine tegmental nucleus in the mediation of locomotion and orofacial stereotypy induced by d-amphetamine and apomorphine in the rat. Neuroscience 58:817–833
Iwamoto ET (1990) Nicotine conditions place preferences after intracerebral administration in rats. Psychopharmacology (Berl) 100:251–257
Jourdain A, Semba K, Fibiger HC (1989) Basal forebrain and mesopontine tegmental projections to the reticular thalamic nucleus: an axonal collateralization and immunohistochemical study in the rat. Brain Res 505:55–65
Keating GL, Winn P (2002) Examination of the role of the pedunculopontine tegmental nucleus in radial maze tasks with or without a delay. Neuroscience 112:687–696
Kita T, Kita H (2011) Cholinergic and non-cholinergic mesopontine tegmental neurons projecting to the subthalamic nucleus in the rat. Eur J Neurosci 33:433–443
Kobayashi Y, Okada K (2007) Reward prediction error computation in the pedunculopontine tegmental nucleus neurons. Ann N Y Acad Sci 1104:310–323
Lanca AJ, Sanelli TR, Corrigall WA (2000) Nicotine-induced fos expression in the pedunculopontine mesencephalic tegmentum in the rat. Neuropharmacology 39:2808–2817
Lodge DJ, Grace AA (2006) The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. P Natl Acad Sci USA 103:5167–5172
Losier BJ, Semba K (1993) Dual projections of single cholinergic and aminergic brainstem neurons to the thalamus and basal forebrain in the rat. Brain Res 604:41–52
Louis M, Clarke PB (1998) Effect of ventral tegmental 6-hydroxydopamine lesions on the locomotor stimulant action of nicotine in rats. Neuropharmacology 37:1503–1513
Maclaren DA, Wilson DI, Winn P (2013) Updating of action-outcome associations is prevented by inactivation of the posterior pedunculopontine tegmental nucleus. Neurobiol Learn Mem 102:28–33
MacLaren DA, Markovic T, Clark SD (2014a) Assessment of sensorimotor gating following selective lesions of cholinergic pedunculopontine neurons. Eur J Neurosci 40:3526–3537
MacLaren DA, Santini JA, Russell AL, Markovic T, Clark SD (2014b) Deficits in motor performance after pedunculopontine lesions in rats--impairment depends on demands of task. Eur J Neurosci 40:3224–3236
Manaye KF, Zweig R, Wu D, Hersh LB, De Lacalle S, Saper CB, German DC (1999) Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neuroscience 89:759–770
Mansvelder HD, Keath JR, McGehee DS (2002) Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33:905–919
Martinez-Gonzalez C, Bolam JP, Mena-Segovia J (2011) Topographical organization of the pedunculopontine nucleus. Frontiers in neuroanatomy 5:22
Maskos U (2007) Emerging concepts: novel integration of in vivo approaches to localize the function of nicotinic receptors. J Neurochem 100:596–602
Maskos U (2008) The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol 153(Suppl 1):S438–S445
Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP (2005) Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature 436:103–107
Mena-Segovia J, Bolam JP (2011) Phasic modulation of cortical high-frequency oscillations by pedunculopontine neurons. Prog Brain Res 193C:85–92
Mena-Segovia J, Sims HM, Magill PJ, Bolam JP (2008a) Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J Physiol 586:2947–2960
Mena-Segovia J, Winn P, Bolam JP (2008b) Cholinergic modulation of midbrain dopaminergic systems. Brain Res Rev 58:265–271
Miller AD, Forster GL, Metcalf KM, Blaha CD (2002) Excitotoxic lesions of the pedunculopontine differentially mediate morphine- and d-amphetamine-evoked striatal dopamine efflux and behaviors. Neuroscience 111:351–362
Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK (1995) Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci 15:5859–5869
Okada K, Kobayashi Y (2013) Reward prediction-related increases and decreases in tonic neuronal activity of the pedunculopontine tegmental nucleus. Front Integr Neurosci 7:36
Okada K, Toyama K, Inoue Y, Isa T, Kobayashi Y (2009) Different pedunculopontine tegmental neurons signal predicted and actual task rewards. J Neurosci 29:4858–4870
Olmstead MC, Franklin KB (1994) Lesions of the pedunculopontine tegmental nucleus block drug-induced reinforcement but not amphetamine-induced locomotion. Brain Res 638:29–35
Olmstead MC, Munn EM, Franklin KB, Wise RA (1998) Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci 18:5035–5044
Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF (2006) Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology 184:391–400
Pan WX, Hyland BI (2005) Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci 25:4725–4732
Parker JL, van der Kooy D (1995) Tegmental pedunculopontine nucleus lesions do not block cocaine reward. Pharmacol Biochem Behav 52:77–83
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier Academic Press, Amsterdam, Boston
Reavill C, Stolerman IP (1990) Locomotor activity in rats after administration of nicotinic agonists intracerebrally. Br J Pharmacol 99:273–278
Redgrave P, Gurney K, Reynolds J (2008) What is reinforced by phasic dopamine signals? Brain Res Rev 58:322–339
Reichelt AC, Lin TE, Harrison JJ, Honey RC, Good MA (2011) Differential role of the hippocampus in response-outcome and context-outcome learning: evidence from selective satiation procedures. Neurobiol Learn Mem 96:248–253
Ros H, Magill PJ, Moss J, Bolam JP, Mena-Segovia J (2010) Distinct types of non-cholinergic pedunculopontine neurons are differentially modulated during global brain states. Neuroscience 170:78–91
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80:1–27
Schultz W (2010) Dopamine signals for reward value and risk: basic and recent data. BBF 6:24
Semba K, Fibiger HC (1992) Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol 323:387–410
Semba K, Reiner PB, Fibiger HC (1990) Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience 38:643–654
Steidl S, Miller AD, Blaha CD, Yeomans JS (2011) M(5) muscarinic receptors mediate striatal dopamine activation by ventral tegmental morphine and pedunculopontine stimulation in mice. PLoS ONE 6:e27538
Steidl S, Wang H, Wise RA (2014) Lesions of cholinergic pedunculopontine tegmental nucleus neurons fail to affect cocaine or heroin self-administration or conditioned place preference in rats. PLoS ONE 9:e84412
Taylor CL, Kozak R, Latimer MP, Winn P (2004) Effects of changing reward on performance of the delayed spatial win-shift radial maze task in pedunculopontine tegmental nucleus lesioned rats. Behav Brain Res 153:431–438
Thompson JA, Felsen G (2013) Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision-making task. J Neurophysiol 110:2817–2829
Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K (2009) Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324:1080–1084
Vezina P, McGehee DS, Green WN (2007) Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog Neuropsychopharmacol Biol Psychiatry 31:1625–1638
Wang HL, Morales M (2009) Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci 29:340–358
Whishaw IQ, Cioe JD, Previsich N, Kolb B (1977) The variability of the interaural line vs the stability of bregma in rat stereotaxic surgery. Physiol Behav 19:719–722
Wilson DI, MacLaren DA, Winn P (2009) Bar pressing for food: differential consequences of lesions to the anterior versus posterior pedunculopontine. Eur J Neurosci 30:504–513
Zellner MR, Ranaldi R (2010) How conditioned stimuli acquire the ability to activate VTA dopamine cells: a proposed neurobiological component of reward-related learning. Neurosci Biobehav Rev 34:769–780
Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD (2009) Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA 106:7281–7288
Acknowledgments
This work was supported by a Wellcome Trust grant (081128/Z/06/Z) to PW and a studentship from the School of Psychology and Neuroscience, University of St. Andrews, to DAAM. We would like to thank Dr. Stewart Clark (University at Buffalo, Sate University of New York) for kindly providing the Dtx-UII, Mary Latimer and Dr. Morag Farquhar for histological assistance and advice, and Dr. Nadine Gut and Nicholas Scott for assistance in behavioral testing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MacLaren, D.A.A., Wilson, D.I.G. & Winn, P. Selective lesions of the cholinergic neurons within the posterior pedunculopontine do not alter operant learning or nicotine sensitization. Brain Struct Funct 221, 1481–1497 (2016). https://doi.org/10.1007/s00429-014-0985-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0985-4