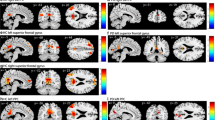
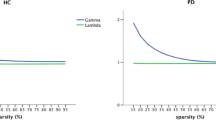
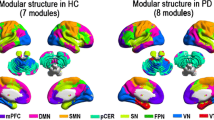
Abstract
Patients with Parkinson’s disease have difficulties with self-initiating a task and maintaining a steady task performance. We hypothesized that these difficulties relate to reorganization in the sensorimotor execution, cingulo-opercular task-set maintenance, and frontoparietal adaptive control networks. We tested this hypothesis using graph theory-based network analysis of a composite network including a total of 86 nodes, derived from the three networks of interest. Resting-state functional magnetic resonance images were collected from 30 patients with Parkinson’s disease (age 42–75 years, 11 females; Hoehn and Yahr score 2–3, average 2.4 ± 0.4) in their off-medication state and 30 matched control subjects (age 44–75 years, 10 females). For each node, we calculated strength as a general measure of connectivity, global efficiency and betweenness centrality as measures of functional integration, and clustering coefficient and local efficiency as measures of functional segregation. We found reduced node strength, clustering, and local efficiency in sensorimotor and posterior temporal nodes. There was also reduced node strength and betweenness centrality in the dorsal anterior insula and temporoparietal junction nodes of the cingulo-opercular network. These nodes are involved in integrating multimodal information, specifically related to self-awareness, sense of agency, and ultimately to intact perception of self-in-action. Moreover, we observed significant correlations between global disease severity and averaged graph metrics of the whole network. In addition to the well-known task-related frontostriatal mechanisms, we propose that the resting-state reorganization in the composite network can contribute to problems with self-initiation and task-set maintenance in Parkinson’s disease.

Similar content being viewed by others
References
Beatty WW, Monson N (1990) Problem solving in Parkinson’s disease: comparison of performance on the Wisconsin and California Card Sorting Tests. J Geriatr Psychiatry Neurol 3(3):163–171
Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain 2124(Pt 11):2131–2146
Blanke O, Mohr C, Michel CM, Pascual-Leone A, Brugger P, Seeck M, Landis T, Thut G (2005) Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. J Neurosci 25(3):550–557
Braak H, Bohl JR, Müller CM, Rüb U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 221(12):2042–2051
Brown P (2000) Cortical drives to human muscle: the piper and related rhythms. Prog Neurobiol 60(1):97–108
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10(3):186–198. doi:10.1038/nrn2575
Catalan MJ, Ishii K, Honda M, Samii A, Hallett M (1999) A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain 122(Pt 3):483–495
Chang LJ, Yarkoni T, Khaw MW, Sanfey AG (2013) Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex 23(3):739–749. doi:10.1093/cercor/bhs065
Christopher L, Marras C, Duff-Canning S, Koshimori Y, Chen R, Boileau I, Segura B, Monchi O, Lang AE, Rusjan P, Houle S, Strafella AP (2014) Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson’s disease with mild cognitive impairment. Brain 137(Pt 2):565–575. doi:10.1093/brain/awt337
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001) Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain 124(Pt 12):2503–2512
Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM (2002) Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain 125(Pt 3):584–594
Cowie D, Limousin P, Peters A, Hariz M, Day BL (2012) Doorway-provoked freezing of gait in Parkinson’s disease. Mov Disord 27(4):492–499. doi:10.1002/mds.23990
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29(3):162–173
Craig AD (2009) How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10(1):59–70. doi:10.1038/nrn2555
Dagher A, Owen AM, Boecker H, Brooks DJ (2001) The role of the striatum and hippocampus in planning: a PET activation study in Parkinson’s disease. Brain 124(Pt 5):1020–1032
Davidsdottir S, Cronin-Golomb A, Lee A (2005) Visual and spatial symptoms in Parkinson’s disease. Vision Res 45(10):1285–1296
Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE (2006) A core system for the implementation of task sets. Neuron 50(5):799–812
Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104(26):11073–11078
Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR Jr, Barch DM, Petersen SE, Schlaggar BL (2010) Prediction of individual brain maturity using fMRI. Science 329(5997):1358–1361. doi:10.1126/science.1194144
Dubois B, Pillon B (1997) Cognitive deficits in Parkinson’s disease. J Neurol 244(1):2–8
Fahn S (1995) The freezing phenomenon in parkinsonism. Adv Neurol 67:53–63
Fahn S, Elton R (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M (eds) Recent developments in Parkinson’s disease. MacMillan Health Care Information, New Jersey, pp 153–163
Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP (2013) Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci 6:80. doi:10.3389/fnsys.2012.00080
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Giese MA, Poggio T (2003) Neural mechanisms for the recognition of biological movements. Nat Rev Neurosci 4(3):179–192
Grahn JA, Parkinson JA, Owen AM (2008) The cognitive functions of the caudate nucleus. Prog Neurobiol 86(3):141–155. doi:10.1016/j.pneurobio.2008.09.004
Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ (2012) Resting state functional connectivity of the striatum in Parkinson’s disease. Brain 135(Pt 12):3699–3711. doi:10.1093/brain/aws281
Hallett M (2003) Parkinson revisited: pathophysiology of motor signs. Adv Neurol 91:19–28
Haslinger B, Erhard P, Kämpfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO (2001) Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 124(Pt 3):558–570
Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I (2010) Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb Cortex 20(5):1175–1186. doi:10.1093/cercor/bhp178
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, Fox PT, Eickhoff SB (2014) The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp 35(6):2741–2753. doi:10.1002/hbm.22363
Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ (2001) What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. 1992. Neurology 57(10 Suppl 3):S34–S38
Iansek R, Huxham F, McGinley J (2006) The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord 21(9):1419–1424
Iturria-Medina Y, Sotero RC, Canales-Rodríguez EJ, Alemán-Gómez Y, Melie-García L (2008) Studying the human brain anatomical network via diffusion-weighted MRI and Graph Theory. Neuroimage 40(3):1064–1076. doi:10.1016/j.neuroimage.2007.10.060
Jacomy M, Venturini T, Heymann S, Bastian M (2014) ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS One 9(6):e98679. doi:10.1371/journal.pone.0098679
Jastorff J, Clavagnier S, Gergely G, Orban GA (2011) Neural mechanisms of understanding rational actions: middle temporal gyrus activation by contextual violation. Cereb Cortex 21(2):318–329. doi:10.1093/cercor/bhq098
Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW (2010) Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 52(2):571–582. doi:10.1016/j.neuroimage.2010.04.246
Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, Saad ZS (2013) Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state FMRI. J Appl Math. doi:10.1155/2013/935154
Kish SJ, Shannak K, Hornykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med 318(14):876–880
Kucyi A, Moayedi M, Weissman-Fogel I, Hodaie M, Davis KD (2012) Hemispheric asymmetry in white matter connectivity of the temporoparietal junction with the insula and prefrontal cortex. PLoS One 7(4):e35589. doi:10.1371/journal.pone.0035589
Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB (2010) A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 214(5–6):519–534. doi:10.1007/s00429-010-0255-z
Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM (2005) Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia 43(6):823–832
Luo C, Song W, Chen Q, Zheng Z, Chen K, Cao B, Yang J, Li J, Huang X, Gong Q, Shang HF (2014) Reduced functional connectivity in early-stage drug-naive Parkinson’s disease: a resting-state fMRI study. Neurobiol Aging 35(2):431–441. doi:10.1016/j.neurobiolaging.2013.08.018
Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A (2004) Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci 24(3):702–710
Nahab FB, Kundu P, Gallea C, Kakareka J, Pursley R, Pohida T, Miletta N, Friedman J, Hallett M (2011) The neural processes underlying self-agency. Cereb Cortex 21(1):48–55. doi:10.1093/cercor/bhq059
Nakamura T, Ghilardi MF, Mentis M, Dhawan V, Fukuda M, Hacking A, Moeller JR, Ghez C, Eidelberg D (2001) Functional networks in motor sequence learning: abnormal topographies in Parkinson’s disease. Hum Brain Mapp 12(1):42–60
Nantel J, McDonald JC, Tan S, Bronte-Stewart H (2012) Deficits in visuospatial processing contribute to quantitative measures of freezing of gait in Parkinson’s disease. Neuroscience 221:151–156. doi:10.1016/j.neuroscience.2012.07.007
Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW (1993) Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain 116(Pt 5):1159–1175
Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC (1998) Abnormal basal ganglia outflow in Parkinson’s disease identified with PET. Implications for higher cortical functions. Brain 121(Pt 5):949–965
Pyles JA, Garcia JO, Hoffman DD, Grossman ED (2007) Visual perception and neural correlates of novel ‘biological motion’. Vision Res 47(21):2786–2797
Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, Passingham R (2002) Attention to action in Parkinson’s disease: impaired effective connectivity among frontal cortical regions. Brain 125(Pt 2):276–289
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52(3):1059–1069. doi:10.1016/j.neuroimage.2009.10.003
Rudie JD, Brown JA, Beck-Pancer D, Hernandez LM, Dennis EL, Thompson PM, Bookheimer SY, Dapretto M (2012) Altered functional and structural brain network organization in autism. Neuroimage Clin 2:79–94. doi:10.1016/j.nicl.2012.11.006
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012) Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2(1):25–32. doi:10.1089/brain.2012.0080
Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O (2000) Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 123(Pt 2):394–403
Salenius S, Avikainen S, Kaakkola S, Hari R, Brown P (2002) Defective cortical drive to muscle in Parkinson’s disease and its improvement with levodopa. Brain 125(Pt 3):491–500
Samuel M, Ceballos-Baumann AO, Blin J, Uema T, Boecker H, Passingham RE, Brooks DJ (1997) Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain 120(Pt 6):963–976
Schultz J, Imamizu H, Kawato M, Frith CD (2004) Activation of the human superior temporal gyrus during observation of goal attribution by intentional objects. J Cogn Neurosci 16(10):1695–1705
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9):2349–2356
Sowden S, Catmur C (2013) The role of the right temporoparietal junction in the control of imitation. Cereb Cortex (Epub ahead of print) PubMed PMID: 24177989
Sperduti M, Delaveau P, Fossati P, Nadel J (2011) Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain Struct Funct 216(2):151–157. doi:10.1007/s00429-010-0298-1
Sporns O, Honey CJ, Kötter R (2007) Identification and classification of hubs in brain networks. PLoS One 2(10):e1049
Tinaz S, Schendan HE, Stern CE (2008) Fronto-striatal deficit in Parkinson’s disease during semantic event sequencing. Neurobiol Aging 29(3):397–407
Tsakiris M, Costantini M, Haggard P (2008) The role of the right temporo-parietal junction in maintaining a coherent sense of one’s body. Neuropsychologia 46(12):3014–3018. doi:10.1016/j.neuropsychologia.2008.06.004
van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE (2010) Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci 30(47):15915–15926. doi:10.1523/JNEUROSCI.2874-10.2010
van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, Goñi J, Hulshoff Pol HE, Kahn RS (2013) Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry 70(8):783–792. doi:10.1001/jamapsychiatry.2013.1328
van der Hoorn A, Renken RJ, Leenders KL, de Jong BM (2014) Parkinson-related changes of activation in visuomotor brain regions during perceived forward self-motion. PLoS One 9(4):e95861. doi:10.1371/journal.pone.0095861
Vogeley K, Fink GR (2003) Neural correlates of the first-person-perspective. Trends Cogn Sci 7(1):38–42
Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P (2011a) Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage 55(1):204–215. doi:10.1016/j.neuroimage.2010.11.074
Wu T, Long X, Wang L, Hallett M, Zang Y, Li K, Chan P (2011b) Functional connectivity of cortical motor areas in the resting state in Parkinson’s disease. Hum Brain Mapp 32(9):1443–1457. doi:10.1002/hbm.21118
Xia M, Wang J, He Y (2013) BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 8:e68910
Yu H, Sternad D, Corcos DM, Vaillancourt DE (2007) Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 35(1):222–233
Zalla T, Sirigu A, Pillon B, Dubois B, Grafman J, Agid Y (1998) Deficit in evaluating pre-determined sequences of script events in patients with Parkinson’s disease. Cortex 34(4):621–627
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program. The authors thank Kazumi Iseki and Cecile Gallea for their help with data collection, Pritha Ghosh and Sarah Kranick for their help with patient assessments, Patrick Malone for his help with pilot data analysis, Ziad Saad for his help with data preprocessing, and Devera Schoenberg, M.Sc., for editorial assistance.
Conflict of interest
The authors declare no conflict of interest in relation to the present manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Box
Appendix: Box
Graph metric definitionsFootnote 1
Functional segregation of a neural network refers to its ability for specialized processing within clusters of nodes.
Functional integration is related to a neural network’s ability to bind information efficiently from distributed regions.
Node strength indicates how strongly one node is connected to the rest of the nodes in the network. It is computed as the sum of the weights of the connections that link a node to the rest of the nodes.
Path is the shortest distance (i.e., minimum number of connections) between a node and every other node in the network. Efficiency is inversely related to path length.
Global efficiency is calculated as the inverse of the average shortest path length between all pairs of nodes in the network. It is a measure of functional integration.
Node Betweenness Centrality indicates how central a node is to the communication among other nodes in the network. It is computed as the fraction of all shortest paths in the network that contain a given node. Nodes with high values of betweenness centrality participate in a large number of shortest paths and potentially function as hubs.
Clustering coefficient measures the density of connections between neighboring nodes. It is computed as the number of connections that exist between the nearest neighbors of a node as a proportion of the maximum number of possible connections. High clustering is associated with high local efficiency of information transfer.
Local efficiency reflects how relevant a node is for the communication among neighbors. It is computed as the inverse of the average shortest path connecting all neighbors of a node. It is a nodal measure of the average efficiency within a local neighborhood, and is related to the clustering coefficient.
Rights and permissions
About this article
Cite this article
Tinaz, S., Lauro, P., Hallett, M. et al. Deficits in task-set maintenance and execution networks in Parkinson’s disease. Brain Struct Funct 221, 1413–1425 (2016). https://doi.org/10.1007/s00429-014-0981-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0981-8