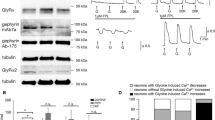
Abstract
Both glycinergic and GABAergic neurons require the vesicular inhibitory amino acid transporter (VIAAT) for synaptic vesicle filling. Presynaptic GABA concentrations are determined by the GABA-synthesizing enzymes glutamate decarboxylase (GAD)65 and GAD67, whereas the presynaptic glycine content depends on the plasma membrane glycine transporter 2 (GlyT2). Although severely impaired, glycinergic transmission is not completely absent in GlyT2-knockout mice, suggesting that other routes of glycine uptake or de novo synthesis of glycine exist in presynaptic terminals. To investigate the consequences of a complete loss of glycinergic transmission, we generated a mouse line with a conditional ablation of VIAAT in glycinergic neurons by crossing mice with loxP-flanked VIAAT alleles with a GlyT2-Cre transgenic mouse line. Interestingly, conditional VIAAT knockout (VIAAT cKO) mice were not viable at birth. In addition to the dominant respiratory failure, VIAAT cKO showed an umbilical hernia and a cleft palate. Immunohistochemistry revealed an almost complete depletion of VIAAT in the brainstem. Electrophysiology revealed the absence of both spontaneous glycinergic and GABAergic inhibitory postsynaptic currents from hypoglossal motoneurons. Our results demonstrate that the deletion of VIAAT in GlyT2-Cre expressing neurons also strongly affects GABAergic transmission and suggest a large overlap of the glycinergic and the GABAergic neuron population during early development in the caudal parts of the brain.










Similar content being viewed by others
References
Aubrey KR, Rossi FM, Ruivo R, Alboni S, Bellenchi GC, Le Goff A, Gasnier B, Supplisson S (2007) The transporters GlyT2 and VIAAT cooperate to determine the vesicular glycinergic phenotype. J Neurosci 27(23):6273–6281. doi:10.1523/JNEUROSCI.1024-07.2007
Bentzen BH, Grunnet M (2011) Central and peripheral GABA(A) receptor regulation of the heart rate depends on the conscious state of the animal. Adv Pharmacol Sci 2011:578273. doi:10.1155/2011/578273
Beyoglu D, Idle JR (2012) The glycine deportation system and its pharmacological consequences. Pharmacol Ther 135(2):151–167. doi:10.1016/j.pharmthera.2012.05.003
Butera RJ Jr, Rinzel J, Smith JC (1999) Models of respiratory rhythm generation in the pre-Botzinger complex. I. Bursting pacemaker neurons. J Neurophysiol 82(1):382–397
Condie BG, Bain G, Gottlieb DI, Capecchi MR (1997) Cleft palate in mice with a targeted mutation in the gamma-aminobutyric acid-producing enzyme glutamic acid decarboxylase 67. Proc Natl Acad Sci USA 94(21):11451–11455
Davies LP, Johnston GA (1974) Postnatal changes in the levels of glycine and the activities of serine hydroxymethyltransferase and glycine:2-oxoglutarate aminotransferase in the rat central nervous system. J Neurochem 22(1):107–112
Delpy A, Allain AE, Meyrand P, Branchereau P (2008) NKCC1 cotransporter inactivation underlies embryonic development of chloride-mediated inhibition in mouse spinal motoneuron. J Physiol 586(4):1059–1075. doi:10.1113/jphysiol.2007.146993
Ding R, Tsunekawa N, Obata K (2004) Cleft palate by picrotoxin or 3-MP and palatal shelf elevation in GABA-deficient mice. Neurotoxicol Teratol 26(4):587–592. doi:10.1016/j.ntt.2004.04.002
Drorbaugh JE, Fenn WO (1955) A barometric method for measuring ventilation in newborn infants. Pediatrics 16(1):81–87
Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR (1998) Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 282(5392):1321–1324
Ferguson C, Hardy S, Werner D, Hileman S, DeLorey T, Homanics G (2007) New insight into the role of the beta3 subunit of the GABAA-R in development, behavior, body weight regulation, and anesthesia revealed by conditional gene knockout. BMC Neurosci 8(1):85
Fujii M, Arata A, Kanbara-Kume N, Saito K, Yanagawa Y, Obata K (2007) Respiratory activity in brainstem of fetal mice lacking glutamate decarboxylase 65/67 and vesicular GABA transporter. Neuroscience 146(3):1044–1052. doi:10.1016/j.neuroscience.2007.02.050
Gomeza J, Hulsmann S, Ohno K, Eulenburg V, Szoke K, Richter D, Betz H (2003a) Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 40(4):785–796
Gomeza J, Ohno K, Hulsmann S, Armsen W, Eulenburg V, Richter DW, Laube B, Betz H (2003b) Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 40(4):797–806
Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL (2001) Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4(9):927–930. doi:10.1038/nn0901-927
Gunther U, Benson J, Benke D, Fritschy JM, Reyes G, Knoflach F, Crestani F, Aguzzi A, Arigoni M, Lang Y et al (1995) Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 92(17):7749–7753
Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ (2001) Disruption of KCC2 reveals an essential role of K–Cl cotransport already in early synaptic inhibition. Neuron 30(2):515–524
Hulsmann S, Oku Y, Zhang W, Richter DW (2000) Metabotropic glutamate receptors and blockade of glial Krebs cycle depress glycinergic synaptic currents of mouse hypoglossal motoneurons. Eur J Neurosci 12(1):239–246
Ishihara N, Armsen W, Papadopoulos T, Betz H, Eulenburg V (2010) Generation of a mouse line expressing Cre recombinase in glycinergic interneurons. Genesis 48(7):437–445. doi:10.1002/dvg.20640
Janczewski WA, Onimaru H, Homma I, Feldman JL (2002) Opioid-resistant respiratory pathway from the preinspiratory neurones to abdominal muscles: in vivo and in vitro study in the newborn rat. J Physiol 545(Pt 3):1017–1026. doi:10.1113/jphysiol.2002.023408
Jo YH (2012) Endogenous BDNF regulates inhibitory synaptic transmission in the ventromedial nucleus of the hypothalamus. J Neurophysiol 107(1):42–49. doi:10.1152/jn.00353.2011
Jursky F, Nelson N (1995) Localization of glycine neurotransmitter transporter (GLYT2) reveals correlation with the distribution of glycine receptor. J Neurochem 64(3):1026–1033. doi:10.1046/j.1471-4159.1995.64031026.x
Jursky F, Nelson N (1996) Developmental expression of the glycine transporters GLYT1 and GLYT2 in mouse brain. J Neurochem 67(1):336–344
Kikuchi G (1973) The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem 1(2):169–187. doi:10.1007/BF01659328
Kling C, Koch M, Saul B, Becker CM (1997) The frameshift mutation oscillator [Glra1(spd-ot)] produces a complete loss of glycine receptor alpha1-polypeptide in mouse central nervous system. Neuroscience 78(2):411–417
Kuwana S, Tsunekawa N, Yanagawa Y, Okada Y, Kuribayashi J, Obata K (2006) Electrophysiological and morphological characteristics of GABAergic respiratory neurons in the mouse pre-Botzinger complex. Eur J Neurosci 23(3):667–674. doi:10.1111/j.1460-9568.2006.04591.x
Lal A, Oku Y, Hulsmann S, Okada Y, Miwakeichi F, Kawai S, Tamura Y, Ishiguro M (2011) Dual oscillator model of the respiratory neuronal network generating quantal slowing of respiratory rhythm. J Comput Neurosci 30(2):225–240. doi:10.1007/s10827-010-0249-0
Latal AT, Kremer T, Gomeza J, Eulenburg V, Hulsmann S (2010) Development of synaptic inhibition in glycine transporter 2 deficient mice. Mol Cell Neurosci 44(4):342–352. doi:10.1016/j.mcn.2010.04.005
Liu P, Jenkins NA, Copeland NG (2003) A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13(3):476–484
Luque JM, Nelson N, Richards JG (1995) Cellular expression of glycine transporter 2 messenger RNA exclusively in rat hindbrain and spinal cord. Neuroscience 64(2):525–535. doi:10.1016/0306-4522(94)00404-S
McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM (1997) Identification and characterization of the vesicular GABA transporter. Nature 389(6653):870–876. doi:10.1038/39908
Medrihan L, Rohlmann A, Fairless R, Andrae J, Doring M, Missler M, Zhang W, Kilimann MW (2009) Neurobeachin, a protein implicated in membrane protein traffic and autism, is required for the formation and functioning of central synapses. J Physiol 587(Pt 21):5095–5106. doi:10.1113/jphysiol.2009.178236
Morgado-Valle C, Baca SM, Feldman JL (2010) Glycinergic pacemaker neurons in preBotzinger complex of neonatal mouse. J Neurosci 30(10):3634–3639. doi:10.1523/JNEUROSCI.3040-09.2010
Nakauchi J, Matsuo H, Kim DK, Goto A, Chairoungdua A, Cha SH, Inatomi J, Shiokawa Y, Yamaguchi K, Saito I, Endou H, Kanai Y (2000) Cloning and characterization of a human brain Na+-independent transporter for small neutral amino acids that transports d-serine with high affinity. Neurosci Lett 287(3):231–235. doi:10.1016/s0304-3940(00)01169-1
Oh WJ, Westmoreland JJ, Summers R, Condie BG (2010) Cleft palate is caused by CNS dysfunction in Gad1 and VIAAT knockout mice. PLoS ONE 5(3):e9758. doi:10.1371/journal.pone.0009758
Onimaru H, Arata A, Homma I (1990) Inhibitory synaptic inputs to the respiratory rhythm generator in the medulla isolated from newborn rats. Pflug Arch 417(4):425–432
Paxinos G (2007) Atlas of the developing mouse brain: at E17.5, PO, and P6. Elsevier, Acad. Press, Amsterdam [u.a.]
Rahman J, Latal AT, Besser S, Hirrlinger J, Hulsmann S (2013) Mixed miniature postsynaptic currents resulting from co-release of glycine and GABA recorded from glycinergic neurons in the neonatal respiratory network. Eur J Neurosci 37(8):1229–1241. doi:10.1111/ejn.12136
Sagne C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B (1997) Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett 417(2):177–183
Saito K, Kakizaki T, Hayashi R, Nishimaru H, Furukawa T, Nakazato Y, Takamori S, Ebihara S, Uematsu M, Mishina M, Miyazaki J, Yokoyama M, Konishi S, Inoue K, Fukuda A, Fukumoto M, Nakamura K, Obata K, Yanagawa Y (2010) The physiological roles of vesicular GABA transporter during embryonic development: a study using knockout mice. Mol Brain 3:40. doi:10.1186/1756-6606-3-40
Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S (2005) ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol 168(6):941–953. doi:10.1083/jcb.200411179
Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G (2009) Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci 12(8):1028–1035. doi:10.1038/nn.2354
Tsunekawa N, Arata A, Obata K (2005) Development of spontaneous mouth/tongue movement and related neural activity, and their repression in fetal mice lacking glutamate decarboxylase 67. Eur J Neurosci 21(1):173–178. doi:10.1111/j.1460-9568.2004.03860.x
Turgeon B, Meloche S (2009) Interpreting neonatal lethal phenotypes in mouse mutants: insights into gene function and human diseases. Physiol Rev 89(1):1–26. doi:10.1152/physrev.00040.2007
Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hubner CA, Represa A, Ben-Ari Y, Khazipov R (2006) Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 314(5806):1788–1792. doi:10.1126/science.1133212
Wallen-Mackenzie A, Gezelius H, Thoby-Brisson M, Nygard A, Enjin A, Fujiyama F, Fortin G, Kullander K (2006) Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J Neurosci 26(47):12294–12307. doi:10.1523/JNEUROSCI.3855-06.2006
Winter SM, Hirrlinger J, Kirchhoff F, Hulsmann S (2007) Transgenic expression of fluorescent proteins in respiratory neurons. Respir Physiol Neurobiol 159(1):108–114. doi:10.1016/j.resp.2007.05.009
Winter SM, Fresemann J, Schnell C, Oku Y, Hirrlinger J, Hulsmann S (2009) Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflug Arch 458(3):459–469. doi:10.1007/s00424-009-0647-1
Wittmeier S, Song G, Duffin J, Poon CS (2008) Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci USA 105(46):18000–18005. doi:10.1073/pnas.0809377105
Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS (2006) A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron 50(4):575–587. doi:10.1016/j.neuron.2006.04.016
Yao D, Mackenzie B, Ming H, Varoqui H, Zhu H, Hediger MA, Erickson JD (2000) A novel system A isoform mediating Na+/neutral amino acid cotransport. J Biol Chem 275(30):22790–22797. doi:10.1074/jbc.M002965200
Zafra F, Aragon C, Olivares L, Danbolt NC, Gimenez C, Storm-Mathisen J (1995a) Glycine transporters are differentially expressed among CNS cells. J Neurosci 15(5 Pt 2):3952–3969
Zafra F, Gomeza J, Olivares L, Aragon C, Gimenez C (1995b) Regional distribution and developmental variation of the glycine transporters GLYT1 and GLYT2 in the rat CNS. Eur J Neurosci 7(6):1342–1352. doi:10.1111/j.1460-9568.1995.tb01125.x
Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bosl MR, Fritschy JM (2005) Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol 482(2):123–141. doi:10.1002/cne.20349
Acknowledgments
This work was funded by the Cluster of Excellence and DFG Research Center Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB). SH and JH received additional support from the DFG (Hu797/7-1, 8-1; Hi1414/2-1). The authors are grateful to Anja-Annett Grützner, Astrid Zeuch, Astrid Ohle and Annette Fahrenholz for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
S. Hülsmann and S. M. Wojcik contributed equally.
Rights and permissions
About this article
Cite this article
Rahman, J., Besser, S., Schnell, C. et al. Genetic ablation of VIAAT in glycinergic neurons causes a severe respiratory phenotype and perinatal death. Brain Struct Funct 220, 2835–2849 (2015). https://doi.org/10.1007/s00429-014-0829-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0829-2