Abstract
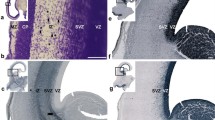
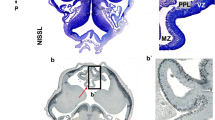
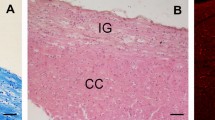
Periventricular pathway (PVP) system of the developing human cerebrum is situated medial to the intermediate zone in the close proximity to proliferative cell compartments. In order to elucidate chemical properties and developing trajectories of the PVP we used DTI in combination with acetylcholinesterase histochemistry, SNAP-25 immunocytochemistry and axonal cytoskeletal markers (SMI312, MAP1b) immunocytochemistry on postmortem paraformaldehyde-fixed brains of 30 human fetuses ranging in age from 10 to 38 postconceptional weeks (PCW), 2 infants (age 1–3 months) and 1 adult brain. The PVP appears in the early fetal period (10–13 PCW) as two defined fibre bundles: the corpus callosum (CC) and the fetal fronto-occipital fascicle (FOF). In the midfetal period (15–18 PCW), all four components of the PVP can be identified: (1) the CC, which at rostral levels forms a voluminous callosal plate; (2) the FOF, with SNAP-25-positive fibers; (3) the fronto-pontine pathway (FPP) which for a short distance runs within the PVP; and (4) the subcallosal fascicle of Muratoff (SFM) which contains cortico-caudate projections. The PVPs are situated medial to the internal capsule at the level of the cortico-striatal junction; they remain prominent during the late fetal and early preterm period (19–28 PCW) and represent a portion of the wider periventricular crossroad of growing associative, callosal and projection pathways. In the perinatal period, the PVPs change their topographical relationships, decrease in size and the FOF looses its SNAP-25-reactivity. In conclusion, the hitherto undescribed PVP of the human fetal cerebrum contains forerunners of adult associative and projection pathways. Its transient chemical properties and relative exuberance suggest that the PVP may exert influence on the development of cortical connectivity (intermediate targeting) and other neurogenetic events such as neuronal proliferation. The PVP’s topographical position also indicates that it is a major site of vulnerability in hypoxic–ischaemic perinatal brain injury.










Similar content being viewed by others
References
Aeby A, Liu Y, de Tiege X, Denolin V, David P, Baleriaux D, Kavec M, Metens T, van Bogaert P (2009) Maturation of thalamic radiations between 34–41 weeks’ gestation: a combined voxel-based study and probabalistic tractography with diffusion tensor imaging. Am J Neuroradiol 30:1780–1786
Aralasmak A, Ulmer JL, Kocak M, Salvan CV, Hillis AE, Yousem DM (2006) Association, commissural, and projection pathways and their functional deficit reported in literature. J Comput Assist Tomogr 30:695–715
Bartelmez GW, Dekaban AS (1962) The early development of the human brain. Contrib Embryol 37:13–32
Bayer SA, Altman J (2002) Atlas of human central nervous system development, vol 1. CRC Press, Boca Raton
Bayer SA, Altman J (2004) Atlas of human central nervous system development series, vol 2. The human brain during the third trimester. CRC Press, Boca Raton
Bayer SA, Altman J (2005) Atlas of human central nervous system development series, vol 3. The human brain during the second trimester. CRC Press, Boca Raton
Bayer SA, Altman J (2006) Atlas of human central nervous system development series, vol 4. The human brain during the late first trimester. CRC Press, Boca Raton
Bayer SA, Altman J (2008) Atlas of human central nervous system development series, vol 5. The human brain during the early first trimester. CRC Press, Boca Raton
Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O’Leary DD (2003) Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol 457:345–360
Bolz J, Uziel D, Mühlfriedel S, Güllmar A, Peuckert C, Zarbalis K, Wurst W, Torii M, Levitt P (2004) Multiple roles of ephrins during the formation of thalamocortical projections: maps and more. J Neurobiol 59:82–94
Bystron I, Blakemore C, Rakic P (2008) Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci 9:110–122
Counsell SJ, Dyet LE, Larkman DJ, Nunes RG, Boardman JP, Allsop JM, Fitzpatrick J, Srinivasan L, Cowan FM, Hajnal JV, Rutherford MA, Edwards AD (2007) Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. Neuroimage 34:896–904
Haydar TF, Wang F, Schwartz ML, Rakic P (2000) Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci 20:5764–5774
His W (1904) Die Entwickelung des menschlichen Gehirns während der ersten Monate. S. Hirzel, Leipzig
Hochstetter F (1919) Beiträge zur Entwicklungsgeschichte des menschlichen Gehirns, 1. Teil. F. Deuticke, Wien und Leipzig
Hoon AH Jr, Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, Campbell ML, Levey E, Mori S, Johnston MV (2009) Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol 51:697–704
Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, Yarowsky P, Donohue P, Graham E, van Zijl PC, Mori S (2006) White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage 33:27–38
Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller MI, Mori S (2009) Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci 29:4263–4273
Hüppi PS, Dubois J (2006) Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med 11:489–497
Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006) DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81:106–116
Johnston MV, Trescher WH, Ishida A, Nakajima W (2001) Neurobiology of hypoxic–ischemic injury in the developing brain. Pediatr Res 49:735–741
Judaš M, Radoš M, Jovanov-Milošević N, Hrabač P, Štern-Padovan R, Kostović I (2005) Structural, immunocytochemical, and mr imaging properties of periventricular crossroads of growing cortical pathways in preterm infants. Am J Neuroradiol 26:2671–2684
Kasprian G, Brugger PC, Weber M, Krssák M, Krampl E, Herold C, Prayer D (2008) In utero tractography of fetal white matter development. Neuroimage 43:213–224
Kim DH, Chung S, Vigneron DB, Barkovich AJ, Glenn OA (2008) Diffusion-weighted imaging of the fetal brain in vivo. Magn Reson Med 59:216–220
Kostović I (1986) Prenatal development of nucleus basalis complex and related fiber systems in man: a histochemical study. Neuroscience 17:1047–1077
Kostovic I, Goldman-Rakic PS (1983) Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol 219:431–447
Kostović I, Jovanov-Milošević N (2006) The development of cerebral connections during the first 20–45 weeks’ gestation. Semin Fetal Neonatal Med 11:415–422
Kostović I, Judaš M (2006) Prolonged coexistence of transient and permanent circuitry elements in the developing cerebral cortex of fetuses and preterm infants. Dev Med Child Neurol 48:388–393
Kostović I, Judaš M (2007) Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev 31:1157–1168
Kostović I, Rakic P (1990) Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297:441–470
Kostović I, Vasung L (2009) Insights from in vitro fetal magnetic resonance imaging of cerebral development. Semin Perinatol 33:220–233
Kostović I, Judaš M, Kostović-Knežević L, Šimić G, Delalle I, Chudy D, Šajin B, Petanjek Z (1991) Zagreb research collection of human brains for developmental neurobiologists and clinical neuroscientists. Int J Dev Biol 35:215–230
Kostović I, Judaš M, Rados M, Hrabac P (2002) Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 12:536–544
Letinic K, Zoncu R, Rakic P (2002) Origin of GABAergic neurons in the human neocortex. Nature 417:645–649
Leviton A, Gressens P (2007) Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci 30:473–477
López-Bendito G, Molnár Z (2003) Thalamocortical development: how are we going to get there? Nat Rev Neurosci 4:276–289
López-Bendito G, Chan CH, Mallamaci A, Parnavelas J, Molnár Z (2002) Role of Emx2 in the development of the reciprocal connectivity between cortex and thalamus. J Comp Neurol 451:153–169
López-Bendito G, Cautinat A, Sánchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marín O, Garel S (2006) Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell 125:127–142
LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR (1995) GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15:1287–1298
Makris N, Papadimitriou GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN (2007) The occipitofrontal fascicle in humans: a quantitative, in vivo, DT-MRI study. Neuroimage 37:1100–1111
Maric D, Liu QY, Maric I, Chaudry S, Chang YH, Smith SV, Sieghart W, Fritschy JM, Barker JL (2001) GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABA(A) autoreceptor/Cl-channels. J Neurosci 21:2343–2360
Mathur A, Inder T (2009) Magnetic resonance imaging—insights into brain injury and outcomes in premature infants. J Commun Disord 42:248–255
Ment LR, Hirtz D, Hüppi PS (2009) Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol 8:1042–1055
Miller SP, Ferriero DM (2009) From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci 32:496–505
Molnár Z, Blakemore C (1995) How do thalamic axons find their way to the cortex? Trends Neurosci 18:389–397
Molnár Z, Butler AB (2002) The corticostriatal junction: a crucial region for forebrain development and evolution. Bioessays 24:530–541
Mori S, Crain BJ, Chacko VP, van Zijl PCM (1999) Three dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269
O’Rahilly R, Müller F (2006) Embryonic human brain: sn atlas of developmental stages, 3rd edn. Wiley, Hoboken
Obersteiner H, Redlich E (1902) Zur Kenntnis des Stratum (Fasciculus) subcallosum (Fasciculus nuclei caudati) und des Fasciculus fronto-occipitalis (reticulirtes cortico-caudales Bündel). Arbeiten aus dem Neurologischen Institut an der Wiener Universität 8:286–307
Petanjek Z, Kostović I, Esclapez M (2009) Primate-specific origins and migration of cortical GABAergic neurons. Front Neuroanat 3:26
Petrides M, Pandya DN (2007) Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci 27:11573–11586
Rakic P (1988) Specification of cerebral cortical areas. Science 241:170–176
Rakic P (2002) Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci 3:65–71
Rakic P, Yakovlev PI (1968) Development of the corpus callosum and cavum septi in man. J Comp Neurol 132:45–72
Rosett J (1933) The myth of the occipitofrontal association tract. Arch Neurol Psychiatry 30:1248–1258
Schmahmann J, Pandya DN (2006) Fiber pathways of the brain. Oxford University Press, Oxford
Schmahmann J, Pandya DN (2007) The complex history of the fronto-occipital fasciculus. J Hist Neurosci 16:362–377
Schmahmann J, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, Wedeen VJ (2007) Association fiber pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653
Schwartz ML, Goldman-Rakic PS (1982) Single cortical neurones have axon collaterals to ipsilateral and contralateral cortex in fetal and adult primates. Nature 299:154–155
Schwartz ML, Goldman-Rakic PS (1984) Callosal and intrahemispheric connectivity of the prefrontal association cortex in rhesus monkey: relation between intraparietal and principal sulcal cortex. J Comp Neurol 226:403–420
Schwartz ML, Goldman-Rakic PS (1991) Prenatal specification of callosal connections in rhesus monkey. J Comp Neurol 307:144–162
Sestan N, Rakic P, Donoghue MJ (2001) Independent parcellation of the embryonic visual cortex and thalamus revealed by combinatorial Eph/ephrin gene expression. Curr Biol 11:39–43
Smart IHM, Dehay C, Giroud P, Berland M, Kennedy H (2002) Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex 12:37–53
Ulfig N, Setzer M, Neudörfer F, Bohl J (2000) Distribution of SNAP-25 in transient neuronal circuitries of the developing human forebrain. Neuroreport 11:1259–1263
Uziel D, Garcez P, Lent R, Peuckert C, Niehage R, Weth F, Bolz J (2006) Connecting thalamus and cortex: the role of ephrins. Anat Rec A Discov Mol Cell Evol Biol 288:135–142
Vasung L, Huang H, Jovanov-Milošević N, Pletikos M, Mori S, Kostović I (2009) Fronto-subcortical connectivity in the human fetal brain: growth of fiber bundles and axonal strata. Poster No. 223.5/B55. 2009 Neuroscience Meeting Planner. Society for Neuroscience, Chicago, IL
Vasung L, Huang H, Jovanov-Milošević N, Pletikos M, Mori S, Kostović I (2010) Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat. doi:10.1111/j.1469-7580.2010.01260.x
Volpe JJ (2003) Cerebral white matter injury of the premature infant—more common than you think. Pediatrics 112:176–180
Volpe JJ (2008) Neurology of the newborn, 5th edn. Saunders Elsevier, Philadelphia
Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110–124
von Monakow C (1905) Gehirnpathologie. Alfred Holder, Wien
Yakovlev PI, Locke S (1961) Limbic nuclei of thalamus and connections of limbic cortex. III. Corticocortical connections of the anterior cingulate gyrus, the cingulum, and the subcallosal bundle in monkey. Arch Neurol 5:364–400
Zhang J, Evans A, Hermoye L, Lee SK, Wakana S, Zhang W, Donohue P, Miller MI, Huang H, Wang X, van Zijl PCM, Mori S (2007) Evidence of slow maturation of the superior longitudinal fasciculus in early childhood by diffusion tensor imaging. Neuroimage 38:239–247
Acknowledgments
This work has been supported by Croatian Ministry of Science, Education and Sport grants No. 108-1081870-1876 (to I.K.), No. 108-1081870-1878 (to M.J.), and Unity Through Knowledge Fund (UKF) grant (Director: I. Kostović).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasung, L., Jovanov-Milošević, N., Pletikos, M. et al. Prominent periventricular fiber system related to ganglionic eminence and striatum in the human fetal cerebrum. Brain Struct Funct 215, 237–253 (2011). https://doi.org/10.1007/s00429-010-0279-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-010-0279-4