Abstract
The cingulate gyrus, as a prominent part of the human limbic lobe, is involved in the integration and regulation of complex emotional, executive, motivational, and cognitive functions, attributed to several functional regions along the anteroposterior axis. In contrast to increasing knowledge of cingulate function in the adult brain, our knowledge of cingulate development is based primarily on classical neuroembryological studies. We aimed to reveal the laminar and cellular development of the various cingulate regions during the critical period from 7.5 to 15 postconceptional weeks (PCW) before the formation of Brodmann type arealization, employing diverse molecular markers on serial histological sections of postmortem human fetal brains. The study was performed by analysis of: (1) deep projection neuron (DPN) markers laminar dynamics, (2) all transient laminar compartments, and (3) characteristic subplate (SP) formation-expansion phase. We found that DPN markers labeling an incipient cortical plate (CP) were the first sign of regional differentiation of the dorsal isocortical and ventral mesocortical belt. Remarkably, increased width of the fibrillar marginal zone (MZ) towards the limbus, in parallel with the narrowing of CP containing DPN, as well as the diminishment of subventricular zone (SVZ) were reliable landmarks of early mesocortical differentiation. Finally, the SP formation pattern was shown to be a crucial event in the isocortical cingulate portion, given that the mesocortical belt is characterized by an incomplete CP delamination and absence of SP expansion. In conclusion, laminar DPN markers dynamics, together with the SVZ size and mode of SP formation indicate regional belt-like cingulate cortex differentiation before the corpus callosum expansion and several months before Brodmann type arealization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The great limbic lobe (le grand lobe limbique) that encircles the brainstem and the great commissure corpus callosum (CC), introduced by French neurologist Paul Broca, and later explored by James Papez and Paul MacLean, was enigmatic since its discovery with little attention paid to the cytoarchitectonic differences in the cingulate gyrus (Broca 1878; Papez 1937; MacLean 1949, 1952; Pessoa and Hof 2015).
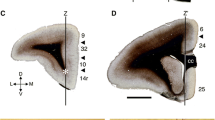
Functional neuroimaging made it obvious that the cingulate gyrus (cingulate cortex) is not a homogenous structure, but that it is comprised of several functional divisions. Vogt introduced the four-region neurobiological model that describes anatomically and functionally different regions of the cingulate cortex and according to this model, it is subdivided into the anterior cingulate cortex (ACC), corresponding to Brodmann areas (BA) 32, 24 and 25; midcingulate cortex (MCC); posterior cingulate cortex (PCC), corresponding to BA 31 and 23 and, retrosplenial cortex (RSC), located posteroinferiorly to the splenium of the CC and cytoarchitectonically referred to as BA 29 and 30 (Vogt 1993, 2009, 2019; Palomero-Gallagher et al. 2008; Vogt and Palomero-Gallagher 2012). We agree that Brodmann cytoarchitectonic maps (Brodmann 1909; Economo and Koskinas 1925) are not suitable for analyzing early regional cortical development, but for analyzing adult brains as stated by Filimonoff (1947). The cingulate gyrus is involved in the integration and regulation of complex emotional (mood states, maternal-infant interactions, social interactions) (Vogt 2005; Gholampour et al. 2020), executive (motor, vocalization, visceromotor) (Carter et al. 1999; Asemi et al. 2015), motivational (Touroutoglou et al. 2019), and cognitive (nociceptive, self-awareness and self-generated decisions) (Paus 2001; Vogt 2005; Maister et al. 2015; Lou et al. 2017; Falsaperla et al. 2022) functions attributed to several functional regions and hubs along the anteroposterior axis (ACC, MCC, and PCC) (Vogt and Palomero-Gallagher 2012; Catani et al. 2013; Rolls 2019). The anterior part of the cingulate gyrus is primarily involved in emotions and autonomic responses, MCC has primarily premotor functions due to its projections to the spinal cord, motor and premotor cortices, while the PCC and RSC are involved in memory and visuospatial orientation (Devinsky et al. 1995; Maddock et al. 2001; Vogt 2009, 2019).
The underlying structural foundation of these distinct functions was explained by differences in the architecture and connectivity of these areas (Palomero-Gallagher et al. 2008; Vogt and Palomero-Gallagher 2012). A review of allocortical portions was presented in great detail in the handbook of Stephan (1975) showing that the major part of the dorsal cingulate gyrus is isocortical (neocortical), first observed by Cajal (1911). Connectivity of the cingulate cortex with diverse cortical and subcortical structures was initially exquisitely shown by Yakovlev et al. (1960a,b,c,d) and Nauta (1972) who proposed the new limbic system concept where cingulate cortex as a part of the frontal lobe, is included in the limbic connectivity centered around hypothalamus (Nauta 1972).
After the introduction of functional neuroimaging, the operational significance of the cingulum fiber bundle, a massive compact white matter tract that runs off the cingulate cortex, was attributed to its contributions to the limbic system, as a variable and weakly defined system of clear clinical interest. The associative cingulum bundle is shown with diffusion tensor imaging (DTI) to be a major hub in the human brain connectome (Vasung et al. 2010; Bubb et al. 2018).
In this paper, the focus is on the role of deep projection neurons-DPN (cortical layer V and VI neurons), born before superficial layer neurons, and the phenomenon of the secondary expansion of the SP (second CP phase, SP formation phase) (Poliakov 1949; Kostovic and Rakic 1990; Duque et al. 2016). Based on our previous studies, we included all layers (compartments) of the future cerebral cortex in the analysis (“compartmental approach”), with a particular emphasis on all transient zones forming a cortical anlage: cortical zones (MZ, CP, SP) and proliferative zones (VZ and SVZ) (Meyer 2001; Bystron et al. 2008; Luhmann et al. 2018; Žunić Išasegi et al. 2018). Therefore, in this study, we selected a period between 7.5 and 15 PCW in the early fetal life when differentiation of the ventral and dorsal cingulate cortex occurs. Currently, there is a gap in knowledge on molecular mechanisms, as well as the cytoarchitectonic descriptions between the early cortex-type territorial parcellation and later-occurring areal differentiation. Importantly, numerous Hominini-specific genes are expressed during that time of development (Nowakowski et al. 2017). Moreover, the role of diverse genes is well examined in the late embryonic period, indicating a huge comparative and evolutionary significance. The late embryonic period requires more attention from the point of revealing expression of transcription factors, extracellular matrix (ECM) molecules, and additional DPN markers, given that supragranular layer neurons are not born yet. Previously, it was shown that limbic supracallosal cortex development occurs in a shorter period (Macchi 1951), and thus is more mature at birth than the lateral telencephalon.
Additionally, this study is also focused on the dorsoventral differentiation of the cingulate cortex before, during, and immediately after CC development. More specifically, we aimed to provide developmental data on the differences in the enigmatic laminar development of the ventral and dorsal portion of the cingulate gyrus in the ACC and PCC during early prenatal development. Previously described cytoarchitectonic differences were further correlated using DPN markers, in addition to glial, synaptic, fibrillar, and ECM markers. Therefore, we analyzed early regional differences in the medial interhemispheric cortex following the dynamics of neuronal laminar markers, i.e., markers of future projection neurons, and thus analyzed CP delamination and SP formation-expansion (Hevner et al. 2001; Bedogni et al. 2010; Duque et al. 2016). Given that dorsal and ventral cingulate gyrus are characterized by anteroposterior and dorsoventral structural and functional differences, one of our aims was to discover how and when these differences appear.
The prenatal development of the nonhomogeneous and complex cingulate gyrus system along the medial aspect of the hemisphere was studied during the early days of histology, as shown in the classical literature (His 1904; Hochstetter 1919; Macchi 1951; Kahle 1969; Stephan 1975). Early developmental differences between the anlage of three-layered archicortex, transitional mesocortex (Filimonoff 1947; Stephan 1975) and six-layered isocortex were described (Rose 1926; Filimonoff 1947; Kahle 1969; Stephan 1975), but the debate remains as to what is the major substrate of these differences. Certainly, it was shown that the MZ and CP width serves as a key landmark in distinguishing the cingulate gyrus before the appearance of the cingulate sulcus, CC, and supragranular cortical layers (Rakic and Yakovlev 1968; Kostović and Krmpotić 1976). Additional criteria were later proposed by Kostović et al. (2004), who found differential distribution in synaptic arrangement and neural morphology from the dorsal to the ventral cingulate cortex. Also, Kostović and Krmpotić (1976) proposed that the “second cortical plate” (Poliakov 1949), subplate formation (SPf), and differences in the SP secondary expansion, may explain differences between the ventral mesocortical and the dorsal isocortical parts of the cingulate gyrus (Kostović and Krmpotić 1976; Kostović et al. 2004).
The cingulate cortex develops very early, like other parts of the limbic cortex (Rakic and Nowakowski 1981) and it participates in active resting state networks in utero in vivo (Doria et al 2010) which from early on are involved in behavioral and emotional neural networks. Hence, besides understanding the functional anatomy of the cingulate cortex in the adult human brain, it is crucial to understand the prenatal development of the human cingulate cortex, if we want to understand how it may participate in the later pathogenesis of diverse neurodevelopmental disorders, such as autism spectrum disorder-ASD (Mundy 2003; Simms et al. 2009), obsessive–compulsive disorder and behavior (McGovern and Sheth 2017), sociopathic behavior, diminished self-awareness, bipolar disorder (Kowatch et al. 2003), and schizophrenia (Yücel et al. 2002; Leech and Sharp 2014) among others. The cingulate cortex is an excellent model to study the laminar differential development of basic cortical types (iso-, archi-, meso-cortex).
We hypothesize that DPN markers show cortex-type specific regional differentiation during the early fetal period and the final pattern of differences between main cortical types is established during the SP formation. We expect that simultaneous analysis of proliferative markers in VZ/SVZ and projection neuron markers in CP and SP will give us an answer, at least topographically grounded, on how dorsoventral and anteroposterior differences in the cingulate cortex develop.
These new findings will provide an insight into the early development of the medial interhemispheric cingulate cortex through the early and mid-fetal period (from 7.5 to 15 PCW), with a special emphasis on the SP formation and expansion (13–15 PCW), as a key human specific neurodevelopmental event. Given that the SP formation-expansion period is typical for the six-layered isocortex, we think that only a study focused on the SP formation period can provide an answer to differences in dorsal vs. ventral cingulate cortex development.
Materials and methods
Human fetal brain tissue
Human brain material is part of the Zagreb Neuroembryological Collection (Kostovic et al. 1991), obtained during regular autopsies after spontaneous or medically indicated abortions at clinical hospitals affiliated with the University of Zagreb, School of Medicine. All specimens were without macroscopic or microscopic central nervous system pathology. A sampling of the tissue was performed following the Declaration of Helsinki (2000) and approved by the Internal Review Board of the Ethical Committee of the School of Medicine, University of Zagreb. After extraction during the autopsy, postmortem human brains were immersion-fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS; pH = 7.4). The fetal age was determined based on the pregnancy records and crown-rump length (CRL) in millimeters and expressed in PCWs, instead of gestational weeks (GW). Following fixation, tissue blocks were embedded in paraffin and sectioned in a coronal or semi-horizontal plane on a microtome (Leica, SM2000R, Wetzlar, Germany) at 10 to 20 µm thick sections. In total, seven brain specimens were systematically processed and analyzed in the period from 7.5 to 15 PCW (CRL 28–120 mm). From these seven, three human fetal brain specimens aged 8, 13 and 15 PCW were serially sectioned from the frontal to the occipital pole to follow the anatomy of the whole cingulate gyrus and then processed by immunohistochemistry. The prospective cingulate cortex is located between the folded archicortical dorsal hippocampus ventrally and the isocortex (neocortex) dorsally (Kostović and Krmpotić 1976) and we analyzed the medial interhemispheric cortex.
Immunohistochemistry (IHC) and immunofluorescence (IF) on human postmortem prenatal brain tissue
Classical histological Cresyl violet (Nissl) staining in the 0,5% Cresyl violet solution at room temperature was used to demonstrate different cytoarchitectonic cortical compartments and to allow comparisons of adjacent sections. PAS-Alcian Blue histochemistry was used to demonstrate the abundant ECM predominantly in the SP compartment. Histological stainings were performed according to the previously described protocols (Kostovic and Goldman‐Rakic 1983).
Immunohistochemistry was done according to our standard protocol described by Žunić Išasegi et al. (2018). The first step is deparaffinization of the paraffin-embedded tissue sections in Xylol solution. Following, slides were immersed in 100% EtOH (2 × 5 min), 96% EtOH (2 × 5 min), and 70%EtOH (1 × 5 min) and rinsed in the 1xPBS for 10 min. Antigen retrieval was done by boiling sections in a citrate buffer (pH = 6.0). Following the antigen retrieval process, sections were pretreated with methanol (MetOH) and H2O2 for 30 min to block the endogenous peroxidase activity. Afterward, sections were rinsed and incubated in the blocking solution (5% bovine serum albumin-BSA and 0, 5% Triton X-100 in 1xPBS) for 1 h. Primary antibodies were incubated at 4 °C during the night. We used the following primary antibodies: glial markers anti-GFAP and anti-vimentin, fibrillar anti-SMI312, synaptic and axonal (anti-SNAP25, anti-SYN), projection neuron markers (anti-Tbr1, anti-CTIP2, anti-TLE4, anti-SOX5), ECM marker (anti-NCAN), proliferative (anti-Ki67), stem cell marker (anti-SOX2), a marker of Cajal-Retzius cells (anti-Reelin), intermediate progenitor cell marker (anti-Tbr2) and microtubular marker (anti-TUBB3). For details about primary antibodies, please refer to Table 1. The next day, following rinsing in the 1xPBS, corresponding secondary biotinylated antibodies (Vectastain ABC kit, Vector Laboratories) were used. After rinsing in 1xPBS, sections were incubated with the Avidin–biotin peroxidase complex for 1 h, and staining was visualized with 3,3-diaminobenzidine (DAB) with metal enhancer (Sigma) or without metal enhancer (ImmPACT DAB EqV). Finally, sections were mounted and coverslipped. Immunofluorescence was done according to a standard protocol, similar to IHC, except that secondary antibodies were conjugated with fluorophores (AlexaFluor 555 and AlexaFluor 488), and the TrueBlack quencher (Biotium) was used. Slides were mounted with a mounting medium with nuclear stain DAPI (Vectashield with DAPI) and coverslipped. Images were visualized and analyzed using a high-resolution digital slide scanner NanoZoomer 2.0 RS (Hammamatsu, Japan).
Results
Results are presented in a systematic manner describing all cortical compartments, namely, proliferative zones: VZ and SVZ, and cortical zones: MZ, CP, and SP.
Our focus was on two developmental phases—an early phase before the expansion of the subplate (7.5–10 PCW), and a phase during SP formation—expansion (13–15 PCW). More specifically, we described dorsoventral and anteroposterior differences during the formation of the cingulate cortex using the compartmental approach (analysis of all transient cortical compartments) by Nissl, T-box brain 1 (Tbr1), as well as analyzing additional molecular markers.
Initial differentiation into cortex-type specific cingulate cortex belts-arcs (7.5–10 PCW)
The medial interhemispheric cortex is composed of the dorsal and ventral portion of the cingulate cortex and the hippocampal (archicortical) anlage. The dorsal portion of the prospective cingulate cortex has characteristics of isocortex-neocortex (thick CP and complete SP expansion), while the ventral portion of the cingulate cortex has mesocortical (allocortical) characteristics-thinner CP and SVZ, and incomplete SP expansion.
Regional differences between the dorsal isocortex (neocortex) and medial limbic interhemispheric cortex can already be appreciated during early fetal brain development. Anteroposterior differences of the prospective cingulate gyrus are not yet developed at the earliest stage examined (7.5 PCW) (Fig. 1). At 7.5 PCW apparent transient zones, VZ and preplate (PPL) are observed, even though according to our results, proliferative zones are not yet easily separated. Instead, there is only one broad proliferative zone. Importantly, at this age, the CP is not developed either. It is obvious that the first postmigratory Tbr1 positive cells are already within the PPL, while the cells of the hippocampal (archicortical) anlage are not Tbr1 immunoreactive (Fig. 1).
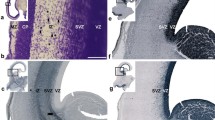
Development of the medial interhemispheric cortex revealed by Nissl staining (a, a’), and Tbr1 immunohistochemistry (b, b’) at 7.5 PCW (Carnegie stage 22), semihorizontal section. a’ and b’ represent the dorsal limbus (prospective cingulate cortex). Cortical plate is not yet developed. The section is tangentional, showing the first postmigratory Tbr1 positive neurons already within the PPL. In the archicortex (hippocampal limbus) no Tbr1 positive cells are observed (black arrow). The connection between the two limbus is marked with red arrow and it may correspond to the tissue bridge described as the precallosal “sling”. At this stage of development, there are no anteroposterior differences in the prospective cingulate cortex. MZ marginal zone, VZ ventricular zone, PPL preplate. Scalebar: 100 μm
The first anteroposterior differences emerge during the formation of the initial CP at 8 PCW (Fig. 2). The future mesocortex shows some transitional features, e.g., less dense initial CP than in the isocortex. However, the presence of the pioneering CP already distinguishes mesocortical from the archicortical portion, characterized by an enormous MZ and the absence of the CP. Note that Tbr1 is mostly expressed in the SVZ and CP in the early fetal phase and evidently the CP is thinner in the mesocortical portion of the cingulate cortex than in the isocortical part (Fig. 2). In addition, the mesocortical part of the cingulate cortex has wider MZ than the isocortical part (Fig. 2a, b). At 8 PCW, Tbr1 is expressed in the SVZ and CP, and CP is gradually thinning towards the archicortex where it is absent (Fig. 3). The potential anatomical border between the mesocortical cingulate cortex and the archicortex could represent the wedge-shaped termination of the SVZ (inner SVZ-iSVZ) where Tbr2 shows diminished expression (Fig. 4). Note that SVZ is present during the early fetal phase (8 PCW) and Tbr2 positive SVZ is narrowing towards the limbus (Fig. 4), while CELF1 (Popovitchenko et al. 2020), delineates the CP and its gradual narrowing in the medial cortex towards its archicortical part (not shown).
Development of the medial interhemispheric cortex revealed by Nissl staining, Tbr1 and Tbr2 immunohistochemistry at 8 PCW (Carnegie stage 23), coronal section, intermediate level. Tbr1 (d, e, f), projection neuron marker is expressed in the CP and SVZ. Tbr2 (g, h, i), an intermediate progenitor cells marker is predominantly expressed in the SVZ intermediate progenitors. The archicortex (c) has typically enlarged MZ (red asterisk) and underdeveloped CP. Thinner SVZ ventrally in the archicortical portion (hippocampal anlage) is serving as a crucial delineation landmark of neocortex, mesocortex and archicortex. The termination of the SVZ (diminished Tbr2 expression) in the archicortex (black line, i) marks the potential border between the meso- and archi-cortex. MZ marginal zone, CP cortical plate, PSP presubplate, SVZ subventricular zone, VZ ventricular zone, A dorsal neocortex, B mesocortex, C archicortex. Scalebar: 100 μm
Dorsoventral gradient of the Tbr1 expression at 8 PCW (Carnegie stage 23), coronal section, intermediate level. Please note that this figure is the same section as Fig. 2 with greater details. Tbr1 is predominantly expressed in the CP and SVZ at 8 PCW. CP is already developed in neocortex, but not in the archicortex. In the archicortex, MZ is enlarged (red asterisk) and the VZ and SVZ are gradually thinner (red arrow). MZ marginal zone, CP cortical plate, SVZ subventricular zone, VZ ventricular zone. Scalebar: 500 μm
Thinning of the SVZ towards the archicortical limbus at 8 PCW (Carnegie stage 23), coronal section, intermediate level. Tbr2 positive SVZ (green) is narrowing towards the limbus. Ki67 positive cells (red) are observed in the proliferative zones and enlarged archicortical MZ (d). MZ marginal zone, CP cortical plate, SVZ subventricular zone, VZ ventricular zone. Scalebar: 100 μm
The ventral border of the prospective cingulate cortex can be roughly estimated due to significant enlargement of the MZ, underdeveloped CP, and less voluminous VZ and SVZ (iSVZ). SVZ (iSVZ) is shown by the intermediate progenitor cells marker Tbr2 (Fig. 4). Archicortex has no visible SVZ, but shows abundant Tbr2 positive cells (Fig. 4d). Proliferative marker Ki67 is expressed in the typically enlarged archicortical MZ suggesting in situ cellular proliferation in the MZ (Fig. 5). In addition, we observed SOX2 positive stem cells predominantly in the VZ, whereas a proliferative marker Ki67 is expressed in the proliferative zones and MZ (Figs. 4 and 5).
Proliferation in the medial interhemispheric cortex revealed by a proliferative marker Ki67 at 8 PCW, coronal section, intermediate level. Ki67 expression is extensive in the proliferative VZ and SVZ due to intense mitotic activity. In the hippocampal anlage (d, e), Ki67 positive cells (red arrows) are observed in the typically enlarged MZ, suggesting in situ proliferation in the archicortical MZ (d). Archicortical characteristics include the absence of the CP and SVZ, in addition to Ki67 positive proliferative cells in the MZ. SOX2 positive (green) stem cells are predominantly in the VZ (e). MZ marginal zone, CP cortical plate, SVZ subventricular zone, VZ ventricular zone. Scalebar: 500 μm, 100 μm
In the medial interhemispheric cortex at 9 PCW (Fig. 6), CP and SVZ become thinner in the dorsoventral gradient towards the limbus. According to our findings, thinning of the CP, PSP, and SVZ and typical MZ enlargement could delineate the transitional mesocortical anlage of the cingulate cortex. At 10 PCW, Tbr1 positive CP is located between the two fibrillar/synaptic layers (MZ and PSP/IZ), narrowed in the medial limbic cortex, and absent in the archicortex (not shown).
Medial interhemispheric cortical development revealed by Nissl staining and Tbr1 immunohistochemistry at 9 PCW, coronal sections. All transient cortical compartments (MZ, CP, PSP, SVZ, VZ) are easily distinguished. In the medial interhemispheric cortex, CP and SVZ are becoming gradually thinner towards the archicortex (a, b). Tbr1 (a’, b’) is expressed in the CP and SVZ. Please note a broken continuity between two parts of the medial cortex. MZ marginal zone, CP cortical plate, PSP presubplate, SVZ subventricular zone, VZ ventricular zone. Scalebar: 100 μm
Regional ventrodorsal and anteroposterior differences between specific cortical patterns became established during the subplate expansion period (13–15 PCW)
The isocortical (neocortical) SP zone is formed by the secondary expansion of CP cells between 13 and 15 PCW (Duque et al. 2016). Here, regional differences in the SP formation process are observed with Tbr1 marker between the dorsal isocortex and medial interhemispheric cortex composed of the dorsal isocortical and ventral mesocortical portion of the cingulate cortex (Fig. 7). Using Tbr1 immunohistochemistry, we observed the absence of CP delamination (secondary expansion of SP) in the archicortex (Fig. 7). Thus, our main findings reveal how to anatomically differentiate and delineate the dorsal isocortical part of the cingulate gyrus, ventral allocortical-mesocortical part of the cingulate gyrus, and the archicortex (future indusium griseum). The neocortex is characterized by the complete “second” cortical plate (Poliakov 1949) formation and consequently, complete SP formation from the deeper portions of the CP. We correlated these neuroanatomical findings with DPN marker Tbr1 (Figs. 7, 13). Remarkably, the mesocortical part of the cingulate cortex is characterized by the incomplete “second” plate formation; on the contrary, the archicortex has no “second” plate nor the SP formation (Figs. 7, 8). At 13 PCW, CC is formed in the anterior brain portions and it is seen as fibers between iSVZ and oSVZ. The tissue bridge between the two cerebral hemispheres which may correspond to the precallosal “sling” is visible only in some planes of the earliest specimen examined in our study (Shu and Richards 2001).
Subplate formation period revealed by the deep projection neuron marker Tbr1 at 13 PCW, coronal sections, anterior part. Rectangles a, b, c, and d represent the dorsal isocortex (a), medial isocortex (b) and parts of the cingulate cortex (c, d) with magnified images. SP is formed from the deep portion of the CP in the dorsal isocortex, as well as in the dorsal isocortical part of the cingulate cortex (marked with red asterisk). Incomplete SP expansion is observed in the mesocortical ventral cingulate cortex (red arrow), while the archicortical part is characterized by the absence of CP delamination-absence of SP expansion (black arrow). Black lines define the borders between complete, incomplete and absent SP expansion (CP delamination). Corpus callosum is formed, seen as callosal fibers between iSVZ and oSVZ. MZ marginal zone, CP cortical plate, SPf subplate in formation, SVZ subventricular zone, iSVZ inner SVZ, oSVZ outer SVZ, VZ ventricular zone, CC corpus callosum. Scalebar: 100 μm
Dorsal and ventral cingulate cortex characteristics (coronal sections, intermediate part—level of the hippocampus) revealed by Nissl staining (a, b, c) and Tbr1 immunohistochemistry (d, e, f, g). Coronal Nissl-stained section at 13 PCW at the level of the hippocampus (a). Magnification from the rectangle shows dorsal cingulate cortex (dorsal pattern) and prospective cingulate sulcus (red arrow) (b). Laminar architecture of neocortical dorsal cingulate cortex (c). Coronal section at 13 PCW at the level of the hippocampus shown with Tbr1 marker (d). Magnification from the rectangle (e) shows the dorsal (f) and ventral (g) portion of the cingulate cortex. Laminar architecture of the neocortical dorsal cingulate cortex (f). Laminar architecture of the mesocortical ventral cingulate cortex: wide MZ, thinner CP and no SP expansion (g). Please note that the ventral cingulate portion (g) was detached during tissue processing. MZ marginal zone, CP cortical plate, SPf subplate in formation, SVZ subventricular zone, iSVZ inner SVZ, oSVZ outer SVZ, VZ ventricular zone. Scalebar: 100 μm
Notably, proliferative SVZ is thick in the neocortex, narrow in the mesocortical part of the cingulate gyrus, and SVZ is not observed in the archicortex. We suggest that the SVZ thickness is associated with the SP formation and expansion process contributing to differences between isocortical and allocortical development. The SVZ narrowing towards the limbus corresponds to the gradient of diminishment and incomplete SP expansion.
Notably, the cingulate cortex and hippocampal formation have a wide and thick MZ. The enlarged MZ of the archicortical hippocampus shares the characteristics of the neocortical SP, such as intensive synaptogenesis, the enlarged thickness of the zone, and an abundant ECM (Vasung et al. 2010). One of our main findings in the hippocampal formation is that Cornu ammonis (CA) sectors of the hippocampus do not show Tbr1 nor SOX5 expression (Fig. 9d). A sharp border between the isocortex and allocortex where CP expansion is absent was observed in the ventral limbic pallium at 13 PCW (not shown).
Dorsal and ventral laminar pattern of the medial interhemispheric cortex at 13 PCW (coronal sections, intermediate part—level of the hippocampus) revealed by projection neuron marker SOX5. Dorsal pattern (b, c) demonstrates thinning of the CP in the ventral cingulate cortex (SOX5 positive cells). Note that SOX5 marks the border of the mesocortex and archicortex. b: dorsal cingulate cortex, c: ventral cingulate cortex, d: hippocampal formation. MZ marginal zone, CP cortical plate, SPf subplate in formation, SVZ subventricular zone, iSVZ inner SVZ, oSVZ outer SVZ, VZ ventricular zone, G.D. gyrus dentatus, CA cornu ammonis, SUB subiculum. Scalebar: 100 μm
Using DPN markers, such as markers of the future SP neurons and cortical layer VI and V neurons (Bedogni et al. 2010), we further confirmed the differences in the SP expansion period between the isocortical, mesocortical-allocortical portion of the cingulate cortex, and the archicortex (Fig. 9) in a way that the incomplete SP expansion is observed in the ventral cingulate cortex at 13 PCW. As an additional marker to delineate the border between the transitional mesocortical part of the cingulate gyrus and the archicortex we used a DPN marker SOX5 (Fig. 9). An interesting finding was the transitional future retrosplenial cortex (RSC) where cell islands, dissecant layer and deep principal lamina were observed (Fig. 10).
Coronal section through the posterior level of the dorsal allocortex. Hippocampal formation is visible ventrally. At this level, transition towards parahippocampal allocortical pattern is seen—presence of cell islands, dissecant layer and deep principal lamina. Tbr1 is present homogeneously in the neocortical and mesocortical portion of the cingulate cortex, but not in the parahippocampal pattern (Tbr1 positive cell islands). MZ marginal zone, CP cortical plate, SPf subplate in formation, IZ intermediate zone, iSVZ inner subventricular zone, CC corpus callosum, F fornix. Scalebar: 100 μm
We showed that a transcription factor Tbr1 is a reliable indicator of CP delamination, whether the CP is complete or incomplete, and SP formation-expansion (Figs. 7, 11, 12). Our findings suggest that in addition to other criteria, the grade of the SP expansion is one of the main landmarks for delineating the isocortex from the allocortex during cortical development, especially in the period from 13 to 15 PCW.
Medial interhemispheric cortex of the cingulate gyrus at 14 PCW (coronal sections, intermediate part) revealed by diverse molecular markers (a-l): projection neuron markers Tbr1 (b), CTIP2 (d), TLE4 (e) and SOX5 (f), synaptic markers SYN (j) and SNAP25 (k), ECM marker NCAN (g), Vim (h) and GFAP (i) as intermediate filament proteins and markers of radial glia, and fibrillar marker SMI312 (l). Thinning of the CP is observed in the dorso-ventro-medial gradient. The termination of the CP in the archicortex is marked with black arrow (b, e, f). Future indusium griseum is marked with red arrow (a). Please note that gyrification has not started yet, these are tissue fixation artifacts. Scalebar: 100 μm
Medial interhemispheric cortex at 15 PCW (coronal sections, posterior level) revealed by projection neuron markers. Topographical relations of the cingulate cortex resembling later fetal topography for the first time during development (appearance of the cingulate sulcus and sulcus of the corpus callosum). Mesocortical portion of the cingulate cortex is characterized by a wide MZ, thinner Tbr1 positive CP and gradually thinner SVZ. Above the corpus callosum, archicortex (future indusium griseum) is observed. Deep projection neuron marker, Tbr1 (a, d), demonstrates the differences in SP expansion of the neocortex, mesocortex (SP expansion is incomplete) and archicortex (SP expansion is absent). SOX5 (b, c), marker of cortical layer V neurons, serves to delineate neurons of the layer V, as a key element of the cingulate cortex. Ki67 marks proliferative cells. SOX2, stem cell marker, is expressed in the VZ and SVZ (e). MZ marginal zone, CP cortical plate, SPf subplate in formation, SVZ subventricular zone, VZ ventricular zone, CC corpus callosum, CS cingulate sulcus, SCC sulcus of corpus callosum, IG indusium griseum. Scalebar: 100 μm
At 15 PCW, topographical relations resemble late fetal period topography (> 24 PCW) of the cingulate gyrus for the first time during cortical development (Fig. 12). Cingulate sulcus, neocortex with enlarged SP expansion, sulcus of the CC, and the transitional mesocortex in the depth of the sulcus are observed (Fig. 12). Mesocortex shows enlarged MZ and thinner CP, while SVZ gradually disappears. Above the CC, the archicortex (future indusium griseum) is observed (Fig. 12). It is important to emphasize that the future indusium griseum-archicortical portion of the allocortex covering callosal fibers, can be observed only when CC is already developed (at 10–10,5 PCW in the anterior parts of the brain). Additionally, at 15 PCW, CP and SP are developed and colocalization of DPN markers Tbr1 and CTIP2 is observed in the CP (Fig. 13).
Medial interhemispheric cortex (coronal sections, anterior part) at 15 PCW shown with Nissl staining (a) and molecular markers (b–h) on adjacent sections. All transient cortical compartments are present. Black arrow shows prospective cingulate sulcus. Projection neuron markers Tbr1 (b) and CTIP2 (c) show future cortical layer V and VI neurons, as well as SP neurons. Colocalization of Tbr1 and CTIP2 (d) is observed in the CP (yellow). Colocalization of Tbr1 and TLE4, projection neuron marker for future cortical layers VI and V (g), is observed in the CP (magnification of the CP: (h)). Inner subventricular zone is marked with the black asterisk. MZ marginal zone, CP cortical plate, iSVZ inner subventricular zone, VZ ventricular zone, CC corpus callosum, CS cingulate sulcus. Scalebar: 100 μm
In conclusion, we delineated the dorsal from the ventral cingulate cortex (isocortical from mesocortical-allocortical) in a way that the ventral cingulate cortex is characterized by the thinner CP, incomplete formation-expansion of the SP, and typically enlarged MZ (Figs. 7, 9, 14). We showed these cytoarchitectonic differences of the SP expansion (CP delamination) with DPN markers (Tbr1, CTIP2, TLE4, SOX5), while the narrowed SVZ towards the limbus was shown with an intermediate progenitor marker Tbr2 and stem cell marker SOX2. The width of the fibrillar MZ in the mesocortical part of the cingulate cortex is increasing towards the limbus, while DPN containing CP is narrowing together with the disappearance of the PSP zone and gradual diminishment of the SVZ (Fig. 14). According to our results, SP formation-expansion pattern is a crucial event in the isocortical part of the cingulate cortex, while the main characteristic of the mesocortical belt is an incomplete CP delamination and incomplete SP expansion, clearly demonstrated with DPN markers (Fig. 14).
Medial interhemispheric cortex at 15 PCW (coronal sections, posterior part) revealed by a projection neuron marker Tbr1 and a stem cell marker SOX2. Narrowing of the Tbr1 positive CP (green arrow) and SOX2 positive SVZ (red arrow) is observed in the cingulate cortex (a). SOX2, stem cell marker, is expressed in the VZ and SVZ, while projection neuron marker Tbr1 is expressed in the CP and SPf. Higher magnification of the SP formation process reveals the isocortical (b) and mesocortical pattern (c) of SPf. Mesocortical pattern of SPf demonstrates thinning of the CP and SVZ and incomplete SP expansion (c). MZ marginal zone, CP cortical plate, SPf subplate in formation, iSVZ inner subventricular zone, oSVZ outer subventricular zone, VZ ventricular zone. Scalebar: 100 μm
Discussion
Using immunocytochemical DPN visualization and analysis of their differential laminar dynamics in the fetal cerebral compartments, we have shown that the mesocortical, ventral (allocortical) portion of the future cingulate cortex can be distinguished from the larger, dorsal (isocortical) portion of the future cingulate cortex immediately after the formation of the cortical plate at 8 PCW, before the corpus callosum formation. The DPN markers’ expression pattern, as well as analysis of all proliferative, migratory, and differentiation compartments of the cerebral wall (VZ, SVZ, IZ, SP, CP, MZ) during this early fetal period, serves also as a reliable tool in the delineation of the mesocortex apart from the very limbic (dorsal) archicortex. The early laminar DPN settlement suggests that DPN migrate radially along glial fibers (Arellano et al. 2021) from SVZ to the CP and establish regional differences several weeks before interaction with thalamic afferents and several months before cytoarchitectonic areal differentiation of Brodmann’s type (Filimonoff 1947).
During the SP formation between 12 and 14 PCW, the neocortical cingulate cortex portion displayed complete SP expansion, while the mesocortical portion showed incomplete CP delamination and minor SP expansion. We propose this event as a novel criterion in defining the regional geography of the early developing cingulate cortex present several months before differentiation in the Brodmann type of arealization, as described for mature cingulate cortex by Vogt et al. (1987, 1995). Finally, our study supports the findings that molecular and transcription factors regulating the DPN identity in the cingulate cortex, are instrumental during the first trimester of intrauterine life in humans (Kwan et al. 2012; Willsey et al. 2013). Importantly, developmental disorders involving projection neurons and their circuitry may emerge during this critical period. In the following paragraphs, we further discuss these three aspects of our findings.
Early establishment of the cingulate cortex-type specific differentiation by deep projection neuron marker laminar organization
Current studies of initial cortical mantle parcellation in the mammalian cerebrum were focused on the early cortical maps formation (protocortex) finding clear differences between medial (hem) and lateral cortical mantle and establishment of anteroposterior cortical gradient and molecular specification (Rubenstein and Rakic 1999; Fukuchi-Shimogori and Grove 2001; O’Leary et al. 2007; Cadwell et al. 2019). These findings were in accordance with Rakic radial unit hypothesis (Rakic 1988, 2000). Rakic documented that the cell production mosaic in proliferative zones and subsequent organization along radial glia (Arellano et al. 2021) towards CP is a basic principle in the formation of the embryonic columns, thus explaining a gradual cortical maps formation by molecular cellular events in the VZ and SVZ (Nowakowski et al. 2017). According to many studies, Brodmann type of areal specification is formed by the additional influence of cortex-type specific thalamic input (thalamocortical afferents) to cortical neurons (O’Leary et al. 2007; Martini et al. 2018; Simi and Studer 2018; Cadwell et al. 2019).
However, classical neuroembryologists (Rose 1926; Filimonoff 1947; Stephan 1975) showed that in the human cortex, there is a long period (approximately three months) between the initial protomap appearance (Clowry et al. 2018) and the Brodmann maps establishment. During this period, the cortex is developed in basic types (allocortex, isocortex) (Rose 1926, 1927a, 1927b; Filimonoff 1947; Stephan 1975) while the ontogenetic formation of classical cortical maps develops rather late during the preterm period, after the appearance of the six-layered Grundtypus (Kostović and Judaš 2002). This classical concept of early cortex-type specific regions (isocortex, allocortex) differentiation into basic histogenetic subdivisions (Economo and Koskinas 1925; Rose 1926, 1927a, 1928; Filimonoff 1947; Stephan 1975)—“Histogenetische Grundgliederung”, (for review see Stephan (1975)), is supported by our results and may explain how cingulate neocortex and mesocortex appear in belt-like shape along the limbus of the medial interhemispheric mantle, surrounding the dorsal archicortex (choroidal fissure).
First, DPN laminar dynamics show differentiation between mesocortex and neocortex in proliferative (SVZ), migratory (IZ), and postmigratory (CP) cortical compartments. SVZ is thinner in the mesocortex (Kostović and Krmpotić 1976; Pogledic et al. 2021), CP shows wedge-shaped narrowing, while synapse-rich MZ is enlarged towards the archicortex (Kostović and Krmpotić 1976; Kostović et al. 2004). There is no CP that early during development (Kahle 1969; Stephan 1975; Kostović-Knežević et al. 1988; Supèr et al. 1998). We emphasize that this basic cortex-type architecture is “designed” predominantly by the DPN layer, given that superficial projection neurons are not born yet at this early time (Rakic 1974, 1988).
Second, we proposed that the deep CP expansion and SP formation between 13 and 14 PCW can be used as a novel, previously not applied criterion for histogenetic cortex-type specific divisions. The deep loose CP expansion (Kostovic and Rakic 1990; Duque et al. 2016) occurs in the cingulate isocortex in a similar mode as in the dorsolateral neocortex, while the allocortical CP expansion is incomplete, especially at the archicortical border. Important evidence that early cortex-type specification is related to the development of connectivity was presented by Kostović and Krmpotić (1976) and Kostovic et al. (Kostović et al. 2004). Namely, in the neocortex, synapses were predominantly distributed in the MZ and poorly delineated CP, together with the presubplate (PSP) containing only a few synapses. On the other hand, in the dorsal isocortical cingulate cortex, the majority of synapses were present in the deep SP. In the early cingulate cortex, Tbr1 reactivity was present in the whole CP thickness. However, SOX5, a marker of prospective cortical layer V neurons, was present in the superficial portion of the CP, indicating a prospective position of future cortical layer V. Deep expanded CP-SP complex showed a predominance of CTIP2 and TLE4 layer-enriched markers, suggesting common laminar dynamics of layer VI and SP.
Throughout the period examined in the present study, the deep Tbr1 immunoreactive nuclei distribution outlined the shape of the thin CP in the medial cortex in the early fetal phase (8–10 PCW). The most superficial Tbr1 and Reelin immunoreactive neurons of Cajal-Retzius type, nicely show gradual enlargement of the MZ towards the limbic archicortex (Meyer et al. 2000; Bayatti et al. 2008; Alzu’bi and Clowry 2019). However, after the great commissure CC is within the commissural plate, (Hochstetter 1919; Rakic and Yakovlev 1968; Stephan 1975) around 10 PCW (CRL around 50 mm), Tbr1 reactive nuclei were present in the SVZ along with a proliferative cells marker (Ki67), and intermediate progenitors marker (Tbr2). To our knowledge, the period of early CC development between 10 and 14 PCW was not previously described with markers specific to the intermediate progenitors` identity. The most interesting finding was the presence of well-developed SVZ during an early period when DPN are being produced for the limbic cingulate cortex in the primate brain (Rakic 1974; Rakic and Nowakowski 1981). Notably, many layer VI DPN and SP neurons project to the primate-characteristic thalamic associative nuclei, such as the mediodorsal (MD) nucleus (Goldman‐Rakic and Porrino 1985).
Accordingly, it seems logical that the SVZ plays a major role in similarly building primate-characteristic circuitry as proposed for a thoroughly examined role of SVZ during the late second trimester (Rubenstein and Rakic 1999; Hansen et al. 2010; Dehay et al. 2015; Nowakowski et al. 2017). SVZ prominence is an additional excellent indicator of the mesocortical cingulate thickness, which diminishes towards the limbus, parallel with the CC development. Thus, our study further supports the general concept of the SVZ importance for primate cortex formation (Rakic and Nowakowski 1981; Haubensak et al. 2004; Hevner 2007; Kelava et al. 2012; Dehay et al. 2015; Popovitchenko and Rasin 2017; Mora-Bermúdez and Huttner 2022). The fact that the primate characteristic SVZ is producing DPN—predominantly of pyramidal type, shows the tremendous importance of pyramidal neurons for the basic cortical architecture establishment in humans (Cajal 1911; Marin-Padilla 1983; Haydar et al. 2000). This seems to be important for the early cingulate cortex connectivity because pyramidal neurons stretch to the MZ where they form a terminal bouquet in contact with tangential afferents originating from different cortical and subcortical sources (Kostović and Krmpotić 1976; Marín-Padilla 1998). On the other hand, basal dendrites reach deep synaptic stratum and SP neurons dendritic arborization (Bourgeois et al. 1994; Rakic et al. 1994; Kostović 2020).
However, the most enigmatic question regarding thinning of the limbic allocortex is rarely discussed in current studies, including the underlying mechanisms leading to this phenomenon. It is obvious that this limbic (allocortical) portion of the cerebral interhemispheric wall develops in an arc-like fashion above and around a choroidal fissure (Hochstetter 1919; Stephan 1975; O’Rahilly and Müller 2008). It is nicely illustrated on images of medial aspects of hemispheres by Economo and Koskinas (1925), Figure 121 in Stephan`s Handbook (Stephan 1975), Hochstetter models (Hochstetter 1919), and Macchi (Macchi 1951). However, we think that during later development, the crucial morphogenetic factor is the massive interhemispheric callosal fibers formation. First, CC fibers form a barrier for migratory neurons that are produced in the VZ and inner SVZ (iSVZ) and practically split the SVZ. Some of the neurons that do not migrate may stay in the subcallosal gray (Kostović et al. 2002). At the medial limbus of the brain hemisphere, especially during the early developmental period, there may be different signaling molecules acting at the key boundaries between different cell compartments. Therefore, anatomical distinctions might relate to the expression pattern of the transcription factor Lhx2 which is known as a forebrain hem system development regulator (Roy et al. 2014). This mechanism may also be involved in the mesocortical thinning observed in our study. The second proposed mechanism is active signaling and glutamate release in the extracellular space of the VZ/SVZ border that may regulate proliferation (LaMantia 1995). The major objection to this proposal is that during the period of DPN production and migration, the CC is poorly developed, and thus the CC morphogenetic mechanisms may be more important during the second part of gestation (Žunić Išasegi et al. 2018), i.e., during the superficial cortical layers neurons production. By all means, the morphogenetic interactions of signaling molecules such as Lhx2 and ingrowth of callosal axons during changes of proliferative SVZ remain a challenging question for future research.
Subplate formation (expansion) phase is a hallmark of the human cingulate cortex histogenetic subdivisions before onset of arealization
Previous comparative studies of the human and monkey cortex histogenesis have clearly shown that the synapse-rich SP expansion occurring during the early midfetal period is a hallmark of primate neocortical development (Kostovic and Rakic 1990; Duque et al. 2016; Kostović 2020).
In the present study, we have documented that during the SP formation phase in the human cingulate cortex (12–13 PCW), incomplete SP expansion in the transitional mesocortex is an essential cytoarchitectonic and histogenetic criterion for delineating ventral, mesocortical from dorsal, isocortical cingulate cortex. While in the isocortical cingulate cortex SP expansion—deep CP cells “spread down” (Duque et al. 2016) precedes by neocortical mode, the ventral primordial cingulate belt shows incomplete CP “delamination” and SP is extremely narrow at the border with a curved thin cortical sheet of the supracallosal archicortex—future indusium griseum (“dorsal hippocampus”). This new finding via DPN markers confirms histological observation of Kostović and Krmpotić (1976), synaptic distribution analysis in the interhemispheric cortex (Kostović and Krmpotić-Nemanić 1975; Kostović et al. 2004), structural MRI (Bobić-Rasonja et al. 2021), as well as the concept of the thin SP and MZ enlargement as a characteristic of the limbic allocortex (Kostović et al. 1989; Supèr et al. 1998; Šimić et al. 2022). Using the atypical SP formation process in the ventral cingulate cortex, our study demonstrated that before the cingulate sulcus and callosal sulcus formation, mesocortex occupied a large portion of the interhemispheric cortical mantle (His 1904; Hochstetter 1919; Economo and Koskinas 1925), nicely summarized in Figure 121 by Stephan (1975).
The enlarged MZ with massive tangential afferent input and scanty access to subcortical fibers through PSP may explain fewer synapses in the deep cortex of the mesocortical cingulate portion (Kostović and Krmpotić 1976) and other developing allocortical regions (Kostović et al. 1989). In the adult cerebrum, the mesocortical portion remains as a very thin belt-like territory (Stephan 1975; Bobić-Rasonja et al. 2019). With the subsequent cingulate neocortical portion development, the cingulate sulcus appearance, the mesocortical banding, and neuronal production in the late neocortical SVZ, there is a significant increase in isocortical cingulate size and thickness. Parallel development of thalamic and other subcortical afferents, the entrance of collaterals from the thick cingulum bundle, and later cingulate cortex development are dominated by neocortical connectivity mode and neocortical cingulate white matter differentiation (similar to adjacent areas of the interhemispheric neocortex). In parallel with the process of white matter differentiation and SP expansion, there is a gradual differentiation in broad cytoarchitectonic areas corresponding to subgenual, anterior, intermediate, posterior, and retrosplenial portions of the cingulate cortex. This process is less explored and requires future studies implementing novel molecular markers, not only for deep but also for superficial cortical layers, as well as specific markers for unique fields, such as gigantopyramidal field (Braak and Braak 1976) and unique neuronal classes such as von Economo neurons (Allman et al. 2010).
The SP formation timing in our study is in accordance with earlier and faster timing of events in the limbic cortex in comparison with the more dorsal and lateral neocortex. From the study of cortical neuron birthdating (Rakic 1974, 1988), it is obvious that limbic cortical neurons production begins earlier and finishes faster than neurons from dorsal and lateral neocortex. This phenomenon may be related to the early cingulate cortex involvement in visceral, behavioral, and emotional networks that are expressed already in the human newborn, essential for survival and newborn-mother interaction. It also means that the critical vulnerable period for fine developmental limbic cortex lesions occurs earlier than for the neocortex.
The developing cingulate cortex circuitry abnormalities underlying neurodevelopmental disorders
Our results show that Tbr1 immunoreactive, presumably glutamatergic DPN are the main architectural and circuitry elements in both isocortical and mesocortical belts in the early human fetal cingulate cortex. During this period, characterized by the intense proliferation in the SVZ, migration, and laminar DPN positioning dynamics, one may expect increased early cingulate circuitry vulnerability leading to a developmental disorder such as ASD. In support of this finding, it is evident that the set of high confidence autism spectrum disorder (hcASD) genes converge (Willsey et al. 2013) on glutamatergic projection neurons in layers V and VI of the human midfetal PFC (Willsey et al. 2013; Bakken et al. 2016). The most connected hcASD gene within this period is found to be Tbr1 (Willsey et al. 2013). Additionally, it is supported by the data highlighting the Tbr1-regulated network of ASD genes in the developing neocortex (Notwell et al. 2016). Somewhat earlier appearance of Tbr1 immunoreactivity in ACC than reported for the medial frontal cortex by Willsey et al. (2013) may be explained by precocious and faster ACC development. Besides the identification of coexpression networks for deep cortical projection neurons, it is essential to define their involvement in synaptic circuitry. The existence of early cingulate synaptic circuitry may also be a possible substrate of abnormalities caused by genetic factors, as well as external influences including hypoxia, ischemia and other prenatal etiological factors. Unfortunately, previous neuropathological studies rarely paid enough attention to possible fine developmental cingulate cortex lesions (Hammarberg 1893; Triarhou 2020), because cingulate functions were not explored during the time of classical neuroembryological cingulate cortex development studies.
We point out that early fronto-cingulate growth and path forming (Vasung et al. 2010) are possible factors in the critical vulnerability period delineation. It is rather surprising that the most common developmental abnormality, CC agenesis, is analyzed for fiber bundles abnormalities (Barkovich and Norman 1988; Koester and O`Leary 1994; Jovanov-Milošević et al. 2009; Edwards et al. 2014), but very little attention was paid to cingulate architecture alternations (Utsunomiya et al. 2006). Possible cingulate architecture abnormalities related to abnormal callosal development are based on the evidence that an expanded deep part of the developing cingulate cortex-SP also sends axons into the CC (Koester and O`Leary 1994; deAzevedo et al. 1997; Jovanov-Milošević et al. 2009).
It remains to be explored whether, when and how early developmental projection neuron lesions in ACC, MCC and PCC may induce changes in the thalamus. Interstitial white matter neurons, subplate neurons (SPN) derivatives, also project to the thalamus (Goldman‐Rakic and Porrino 1985). For understanding the human developmental disorders mechanisms, the PCC and RSC connection, and the connection with the precuneus, related to human characteristic functions such as self-awareness, are all of the special interest (Cavanna and Trimble 2006; Utevsky et al. 2014). In addition, cingulate circuitry abnormalities may cause nociception abnormalities (Vogt 2005). In the context of normal and abnormal functional cingulate cortex development, it is important to note that cingulate hubs are the backbone of the human structural connectome and resting-state activity appears already in early preterm infants (Sporns et al. 2005; Gao et al. 2009; Rubinov and Sporns 2010; Sporns 2011; van den Heuvel and Sporns 2011; Anderson and Thomason 2013; Thomason et al. 2015; Matthews and Fair 2015; van den Heuvel et al. 2018). This developmental period is beyond the phases we have analyzed in the present study. However, alternations of early basic projection circuitry may influence the later circuitry development in supragranular layers, as well as the interneuronal maturation (Greig et al. 2013). Our study gives normative temporal and spatial parameters for normative cellular and laminar data instrumental to tackle the challenging problem of the pathogenesis of behavioral and cognitive neurodevelopmental disorders.
Data availability
The datasets generated and analyzed during the current study are not publicly available due to ethical reasons but are available from the corresponding author on reasonable request.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- ASD:
-
Autism spectrum disorder
- BA:
-
Brodmann area
- CA:
-
Cornu ammonis
- CC:
-
Corpus callosum
- CP:
-
Cortical plate
- CRL:
-
Crown-rump length
- DPN:
-
Deep projection neurons
- DTI:
-
Diffusion tensor imaging
- ECM:
-
Extracellular matrix
- G.D.:
-
Gyrus dentatus
- GW:
-
Gestational weeks
- hcASD:
-
High confidence ASD genes
- IG:
-
Indusium griseum
- iSVZ:
-
Inner subventricular zone
- IZ:
-
Intermediate zone
- MCC:
-
Midcingulate cortex
- MRI:
-
Magnetic resonance imaging
- MZ:
-
Marginal zone
- oSVZ:
-
Outer subventricular zone
- PCC:
-
Posterior cingulate cortex
- PCW:
-
Postconceptional weeks
- PFC:
-
Prefrontal cortex
- PPL:
-
Preplate
- PSP:
-
Presubplate
- RRID:
-
Research resource identifier
- RSC:
-
Retrosplenial cortex
- SP:
-
Subplate
- SPf:
-
SP in formation
- SPN:
-
Subplate neurons
- SVZ:
-
Subventricular zone
- Tbr1:
-
T-box brain 1
- VZ:
-
Ventricular zone
References
Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR (2010) The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct 214:495–517. https://doi.org/10.1007/s00429-010-0254-0
Alzu’bi A, Clowry GJ, (2019) Expression of ventral telencephalon transcription factors ASCL1 and DLX2 in the early fetal human cerebral cortex. J Anat 235:555–568. https://doi.org/10.1111/joa.12971
Anderson AL, Thomason ME (2013) Functional plasticity before the cradle: a review of neural functional imaging in the human fetus. Neurosci Biobehav Rev 37:2220–2232. https://doi.org/10.1016/j.neubiorev.2013.03.013
Arellano JI, Morozov YM, Micali N, Rakic P (2021) Radial glial cells: new views on old questions. Neurochem Res 46:2512–2524. https://doi.org/10.1007/s11064-021-03296-z
Asemi A, Ramaseshan K, Burgess A, Diwadkar VA, Bressler SL (2015) Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Front Hum Neurosci 9:1–10. https://doi.org/10.3389/fnhum.2015.00309
Bakken TE, Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A (2016) Comprehensive transcriptional map of primate brain development. Nature 535:367–375. https://doi.org/10.1038/nature18637.Comprehensive
Barkovich AJ, Norman D (1988) Anomalies of the corpus callosum: correlation with further anomalies of the brain. Am J Roentgenol 151:171–179. https://doi.org/10.2214/ajr.151.1.171
Bayatti N, Sarma S, Shaw C, Eyre JA, Vouyiouklis DA, Lindsay S, Clowry GJ (2008) Progressive loss of PAX6, TBR2, NEUROD and TBR1 mRNA gradients correlates with translocation of EMX2 to the cortical plate during human cortical development. Eur J Neurosci 28:1449–1456. https://doi.org/10.1111/j.1460-9568.2008.06475.x
Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RAM, Beyer RP, Bammler TK, Rubenstein JLR, Hevner RF (2010) Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci U S A 107:13129–13134. https://doi.org/10.1073/pnas.1002285107
Bobić-Rasonja M, Orešković D, Knezović V, Pogledić I, Pupačić D, Vukšić M, Brugger PC, Prayer D, Petanjek Z, Jovanov-Milošević N (2019) Histological and MRI study of the development of the human indusium griseum. Cereb Cortex 29:4709–4724. https://doi.org/10.1093/cercor/bhz004
Bobić-Rasonja M, Pogledić I, Mitter C, Štajduhar A, Milković-Periša M, Trnski S, Di B, Hainfellner JA, Judaš M, Prayer D, Jovanov-Milošević N (2021) Developmental differences between the limbic and neocortical telencephalic wall: an intrasubject slice-matched 3 T MRI-histological correlative study in humans. Cereb Cortex 31:3536–3550. https://doi.org/10.1093/cercor/bhab030
Bourgeois JP, Goldman-Rakic PS, Rakic P (1994) Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex 4:78–96. https://doi.org/10.1093/cercor/4.1.78
Braak H, Braak E (1976) The pyramidal cells of Betz within the cingulate and precentral gigantopyramidal field in the human brain—a Golgi and pigmentarchitectonic study. Cell Tissue Res 172:103–119. https://doi.org/10.1007/BF00226052
Broca P (1878) Anatomie comparee des circonvolutions cerebrales: le grande lobe limbique et la scissure limbique dans la seire des mammiferes. Rev d` Anthr 1:385–498
Brodmann K (1909) Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth, Leipzig
Bubb EJ, Metzler-Baddeley C, Aggleton JP (2018) The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev 92:104–127. https://doi.org/10.1016/j.neubiorev.2018.05.008
Bystron I, Blakemore C, Rakic P (2008) Development of the human cerebral cortex: boulder committee revisited. Nat Rev Neurosci 9:110–122. https://doi.org/10.1038/nrn2252
Cadwell CR, Bhaduri A, Mostajo-Radji MA, Keefe MG, Nowakowski TJ (2019) Development and arealization of the cerebral cortex. Neuron 103:980–1004. https://doi.org/10.1016/j.neuron.2019.07.009
Cajal SR (1911) Histologie du systeme nerveux de l`homme & des vertebres. Maloine A, Paris
Carter CS, Botvinick MM, Cohen JD (1999) The role of the anterior cingulate cortex in executive processes of cognition. Rev Neurosci 10:49–57
Catani M, Dell’Acqua F, Thiebaut de Schotten M (2013) A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev 37:1724–1737. https://doi.org/10.1016/j.neubiorev.2013.07.001
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583. https://doi.org/10.1093/brain/awl004
Clowry GJ, Alzu’bi A, Harkin LF, Sarma S, Kerwin J, Lindsay SJ (2018) Charting the protomap of the human telencephalon. Semin Cell Dev Biol 76:3–14. https://doi.org/10.1016/j.semcdb.2017.08.033
DeAzevedo L, Hedin-Pereira C, Lent R (1997) Callosal neurons in the cingulate cortical plate and subplate of human fetuses. J Comp Neurol 386:60–70. https://doi.org/10.1002/(SICI)1096-9861(19970915)386:1%3c60::AID-CNE7%3e3.0.CO;2-B
Dehay C, Kennedy H, Kosik KS (2015) The outer subventricular zone and primate-specific cortical complexification. Neuron 85:683–694. https://doi.org/10.1016/j.neuron.2014.12.060
Devinsky O, Morrell MJ, Vogt BA (1995) Contributions of anterior cingulate cortex to behaviour. Brain 118:279–306. https://doi.org/10.1093/brain/118.1.279
Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE et al (2010) Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci U S A 107:20015–20020. https://doi.org/10.1073/pnas.1007921107
Duque A, Krsnik Z, Kostovic I, Rakic P (2016) Secondary expansion of the transient subplate zone in the developing cerebrum of human and nonhuman primates. Proc Natl Acad Sci U S A 113:9892–9897
Economo C, Koskinas GN (1925) Atlas of Cytoarchitectonics of the adult human cerebral cortex. Verlag von Julius Springer, Wien
Edwards TJ, Sherr EH, Barkovich AJ, Richards LJ (2014) Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain 137:1579–1613. https://doi.org/10.1093/brain/awt358
Falsaperla R, Collotta AD, Spatuzza M, Familiari M, Vitaliti G, Ruggieri M (2022) Evidences of emerging pain consciousness during prenatal development: a narrative review. Neurol Sci 43:3523–3532. https://doi.org/10.1007/s10072-022-05968-2
Filimonoff IN (1947) A rational subdivision of the cerebral cortex. Arch Fur Neurol Und Psychiatr 58:296–311
Fukuchi-Shimogori T, Grove EA (2001) Neocortex patterning by the secreted signaling molecule FGF8. Science 294:1071–1074. https://doi.org/10.1126/science.1064252
Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W (2009) Evidence on the emergence of the brain`s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc Natl Acad Sci U S A 106:9931–9932. https://doi.org/10.1073/pnas.0904281106
Gholampour F, Riem MME, van den Heuvel MI (2020) Maternal brain in the process of maternal-infant bonding: review of the literature. Soc Neurosci 15:380–384. https://doi.org/10.1080/17470919.2020.1764093
Goldman-Rakic PS, Porrino LJ (1985) The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol 242:535–560. https://doi.org/10.1002/cne.902420406
Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD (2013) Molecular logic of neocortical projection neuron specification, development and diversity. Nat Rev Neurosci 14:755–769. https://doi.org/10.1038/nrn3586
Hammarberg C (1893) Studier öfver idiotiens klinik och patologi jämte undersökningar af hjärnbarkens normala anatomi, med 7 plan- scher. Almqvist and Wiksell, Uppsala
Hansen DV, Lui JH, Parker PRL, Kriegstein AR (2010) Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464:554–561. https://doi.org/10.1038/nature08845
Haubensak W, Attardo A, Denk W, Huttner WB (2004) Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A 101:3196–3201
Haydar TF, Wang F, Schwartz ML, Rakic P (2000) Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci 20:5764–5774. https://doi.org/10.1523/jneurosci.20-15-05764.2000
Hevner RF (2007) Layer-specific markers as probes for neuron type identity in human neocortex and malformations of cortical development. J Neuropathol Exp Neurol 66:101–109. https://doi.org/10.1097/nen.0b013e3180301c06
Hevner RF, Shi L, Justice N, Hsueh YP, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JLR (2001) Tbr1 regulates differentiation of the preplate and layer 6. Neuron 29:353–366. https://doi.org/10.1016/S0896-6273(01)00211-2
His W (1904) Die Entwicklung des menschlichen Gehirns wahrend der ersten Monate. Verlag von Hirzel, Leipzig
Hochstetter F (1919) Beitrage zur Entwicklungsgeschichte des menschlichen Gehirns. Franz Deuticke, Leipzig
Jovanov-Milošević N, Čuljat M, Kostović I (2009) Growth of the human corpus callosum: modular and laminar morphogenetic zones. Front Neuroanat 3:1–10. https://doi.org/10.3389/neuro.05.006.2009
Kahle W (1969) Die Entwicklung der menschlichen Grosshirnhemisphare. Springer Verlag, Berlin-Heidelberg
Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC, Okano H, Huttner WB, Borrell V (2012) Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset callithrix jacchus. Cereb Cortex 22:469–481. https://doi.org/10.1093/cercor/bhr301
Koester E, O’Leary DDM (1994) Axons of early generated corpus callosum neurons in cingulate cortex pioneer the. J Neurosci 14:6608–6620
Kostović I (2020) The enigmatic fetal subplate compartment forms an early tangential cortical nexus and provides the framework for construction of cortical connectivity. Prog Neurobiol 194:101883. https://doi.org/10.1016/j.pneurobio.2020.101883
Kostović I, Krmpotić-Nemanić J (1975) The comparative analysis of the laminar pattern of the synaptogenesis in limbic and neocortical areas of human fetal cortex. Exp Brain Res 23(Suppl):111
Kostović I, Krmpotić J (1976) Early prenatal ontogenesis of the neuronal connections in the interhemispheric cortex of the human gyrus cinguli. Verh Anat Ges 70:305–316
Kostović I, Judaš M (2002) Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec 267:1–6. https://doi.org/10.1002/ar.10069
Kostović-Knežević L, Kostović I, Judaš M (1988) Fine structural organization of the telencephalic wall before the appearance of the cortical plate: eleven millimeter human embryo. In: Fine structural organization of the telencephalic wall before the appearance of the cortical plate: eleven millimeter human embryo. 81.
Kostović I, Seress L, Mrzljak L, Judaš M (1989) Early onset of synapse formation in the human hippocampus: a correlation with Nissl-Golgi architectonics in 15- and 16.5-week-old fetuses. Neuroscience 30:105–116. https://doi.org/10.1016/0306-4522(89)90357-6
Kostović I, Rašin MR, Petanjek Z, Judaš M (2002) Morphological characteristics of the cells in the subcallosal zone (nucleus septohippocampalis) of the human fetus. Neuroembryology 1:97–104. https://doi.org/10.1159/000063528
Kostović I, Judaš M, Kostović-Knežević L (2004) Distribution of synapses in the human cingulate cortex during the formation of the subplate zone. In: Fourth Forum of European Neuroscience: Abstracts Lisabon: FENS
Kostovic I, Goldman-Rakic PS (1983) Transient cholinesterase staining in the mediodorsal nucleus of the thalamus and its connections in the developing human and monkey brain. J Comp Neurol 219:431–447
Kostovic I, Rakic P (1990) Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol 297:441–470
Kostovic I, Judas M, Kostovic-Knezevic L, Simic G, Delalle I, Chudy D, Sajin B, Petanjek Z (1991) Zagreb research collection of human brains for developmental neurobiologists and clinical neuroscientists. Int J Dev Biol 35:215–230. https://doi.org/10.1387/ijdb.1687658
Kowatch RA, Sethuraman G, Hume JH, Kromelis M, Weinberg WA (2003) Combination pharmacotherapy in children and adolescents with bipolar disorder. Biol Psychiatry 53:978–984. https://doi.org/10.1016/S0006-3223(03)00067-2
Kwan KY, Šestan N, Anton ES (2012) Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development 1546:1535–1546. https://doi.org/10.1242/dev.069963
LaMantia AS (1995) The usual suspects: GABA and glutamate may regulate proliferation in the neocortex. Neuron 15:1223–1225. https://doi.org/10.1016/0896-6273(95)90002-0
Leech R, Sharp DJ (2014) The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32. https://doi.org/10.1093/brain/awt162
Lou HC, Changeux JP, Rosenstand A (2017) Towards a cognitive neuroscience of self-awareness. Neurosci Biobehav Rev 83:765–773. https://doi.org/10.1016/j.neubiorev.2016.04.004
Luhmann HJ, Kirischuk S, Kilb W (2018) The superior function of the subplate in early neocortical development. Front Neuroanat 12:1–14. https://doi.org/10.3389/fnana.2018.00097
Macchi G (1951) The ontogenetic development of the olfactory telencephalon in man. J Comp Neurol 95:245–305. https://doi.org/10.1002/cne.900950203
MacLean PD (1949) Psychosomatic disease and the “visceral brain”: recent developments bearing on the Papez theory of emotion. Psychosom Med 11:338–353
MacLean PD (1952) Some psychiatric implications of physiological studies on frontotemporal portion of limbic system (Visceral brain). Electroencephalogr Clin Neurophysiol 4:407–418. https://doi.org/10.1016/0013-4694(52)90073-4
Maddock RJ, Garrett AS, Buonocore MH (2001) Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104:667–676. https://doi.org/10.1016/S0306-4522(01)00108-7
Maister L, Slater M, Sanchez-Vives MV, Tsakiris M (2015) Changing bodies changes minds: owning another body affects social cognition. Trends Cogn Sci 19:6–12. https://doi.org/10.1016/j.tics.2014.11.001
Marín-Padilla M (1983) Structural organization of the human cerebral cortex prior to the appearance of the cortical plate. Anat Embryol 168:21–40. https://doi.org/10.1007/BF00305396
Marín-Padilla M (1998) Cajal-Retzius cells and the development of the neocortex. Trends Neurosci 21:64–71. https://doi.org/10.1016/S0166-2236(97)01164-8
Martini FJ, Moreno-Juan V, Filipchuk A, Valdeolmillos M, López-Bendito G (2018) Impact of thalamocortical input on barrel cortex development. Neuroscience 368:246–255. https://doi.org/10.1016/j.neuroscience.2017.04.005
Matthews M, Fair DA (2015) Research review: functional brain connectivity and child psychopathology—overview and methodological considerations for investigators new to the field. J Child Psychol Psychiatry Allied Discip 56:400–414. https://doi.org/10.1111/jcpp.12335
McGovern RA, Sheth SA (2017) Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: Converging evidence from cognitive neuroscience and psychiatric neurosurgery. J Neurosurg 126:132–147. https://doi.org/10.3171/2016.1.JNS15601
Meyer G (2001) Human neocortical development: the importance of embryonic and early fetal events. Neuroscientist 7:303–314. https://doi.org/10.1177/107385840100700407
Meyer G, Schaaps JP, Moreau L, Goffinet AM (2000) Embryonic and early fetal development of the human neocortex. J Neurosci 20:1858–1868. https://doi.org/10.1523/jneurosci.20-05-01858.2000
Mora-Bermúdez F, Huttner WB (2022) What are the human-specific aspects of neocortex development? Front Neurosci 16:1–8. https://doi.org/10.3389/fnins.2022.878950
Mundy P (2003) Annotation: the neural basis of social impairments in autism: the role of the dorsal medial-frontal cortex and anterior cingulate system. J Child Psychol Psychiatry Allied Discip 44:793–809. https://doi.org/10.1111/1469-7610.00165
Nauta WJ (1972) Neural associations of the frontal cortex. Acta Neurobiol Exp 32:125–140
Notwell JH, Heavner WE, Darbandi SF, Katzman S, William L, Ortiz-londono CF, Tastad D, Eckler MJ, Rubenstein JLR, Mcconnell SK, Chen B, Bejerano G (2016) Tbr1 regulates autism risk genes in the developing neocortex. Genome Res 26:1013–1022
Nowakowski TJ, Bhaduri A, Pollen AA, Alvarado B, Mostajo-Radji MA, Di Lullo E, Haeussler M, Sandoval-Espinosa C, Liu SJ, Velmeshev D, Ounadjela JR, Shuga J, Wang X, Lim DA, West JA, Leyrat AA, Kent WJ, Kriegstein AR (2017) Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 358:1318–1323. https://doi.org/10.1126/science.aap8809
O’Leary DDM, Chou SJ, Sahara S (2007) Area patterning of the mammalian cortex. Neuron 56:252–269. https://doi.org/10.1016/j.neuron.2007.10.010
O’Rahilly R, Müller F (2008) Significant features in the early prenatal development of the human brain. Ann Anat 190:105–118. https://doi.org/10.1016/j.aanat.2008.01.001
Palomero-Gallagher N, Mohlberg H, Zilles K, Vogt B (2008) Cytology and receptor architecture of human anterior cingulate cortex. J Comp Neurol 508:906–926. https://doi.org/10.1002/cne.21684
Papez JW (1937) A proposed mechanism of emotion. J Neuropsychiatr 7:103–112
Paus T (2001) Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2:417–424. https://doi.org/10.1038/35077500
Pessoa L, Hof PR (2015) From Paul Broca`s great limbic lobe to the limbic system. J Comp Neurol 523:2495–2500. https://doi.org/10.1002/cne.23840.From
Pogledic I, Schwartz E, Bobić-Rasonja M, Mitter C, Baltzer P, Gruber GM, Milković-Periša M, Haberler C, Bettelheim D, Kasprian G, Judaš M, Prayer D, Jovanov-Milošević N (2021) 3T MRI signal intensity profiles and thicknesses of transient zones in human fetal brain at mid-gestation. Eur J Paediatr Neurol 35:67–73. https://doi.org/10.1016/j.ejpn.2021.09.014
Poliakov GI (1949) Structural organization of the human cerebral cortex during ontogenetic development. In: Sarkisov AS, Filimonof IN, Preobrazenskaya NS (eds) Cytoarchitectonics of the cerebral cortex in man, 1st edn. Medgiz, Moscow, pp 33–92
Popovitchenko T, Rasin M (2017) Transcriptional and post-transcriptional mechanisms of the development of neocortical lamination. Front Neuroanat 11:1–19. https://doi.org/10.3389/fnana.2017.00102
Popovitchenko T, Park Y, Page NF, Luo X, Krsnik Z, Liu Y et al (2020) Translational derepression of Elavl4 isoforms at their alternative 5` UTRs determines neuronal development. Nat Commun 11:1674. https://doi.org/10.1038/s41467-020-15412-8
Rakic P (1974) Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 183:425–427
Rakic P (1988) Specification of cerebral cortical areas. Science 241:170–176
Rakic P (2000) Radial unit hypothesis of neocortical expansion. Novartis Found Symp 228:30–45. https://doi.org/10.1002/0470846631.ch3
Rakic P, Yakovlev PI (1968) Development of the corpus callosum and cavum septi in man. J Comp Neurol 132:45–72. https://doi.org/10.1002/cne.901320103
Rakic P, Nowakowski RS (1981) The time of origin of neurons in the hippocampal region of the rhesus monkey. J Comp Neurol 196:99–128. https://doi.org/10.1002/cne.901960109
Rakic P, Bourgeois JP, Goldman-Rakic PS (1994) Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res 102:227–243. https://doi.org/10.1016/S0079-6123(08)60543-9
Rolls ET (2019) The cingulate cortex and limbic systems for emotion, action and memory. Brain Struct Funct 224:3001–3018. https://doi.org/10.1007/s00429-019-01945-2
Rose M (1926) Uber das histogenetische Prinzip der Einteilung der Grosshirnrinde. J Psychol Neurol 32:97–160
Rose M (1927a) Der Allocortex bei Tier und Mensch. J Psychol Neurol 34:1–111
Rose M (1927b) Vergleichende Messungen im Allocortex bei Tier und Mensch. J Psychol Neurol 34:250–255
Rose M (1928) Gyrus limbicus anterior und Regio retrosplenialis (Cortex holoprotoptychos quinquestratificatus). Vergleichende Architektonik bei Tier und Mensch. J Psychol Neurol 35:65–173
Roy A, Gonzalez-Gomez M, Pierani A, Meyer G, Tole S (2014) Lhx2 regulates the development of the forebrain hem system. Cereb Cortex 24:1361–1372
Rubenstein JLR, Rakic P (1999) Genetic control of cortical development. Cereb Cortex 9:521–523. https://doi.org/10.4067/s0718-090x2006000100001
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. https://doi.org/10.1016/j.neuroimage.2009.10.003
Shu T, Richards LJ (2001) Cortical axon guidance by the glial wedge during the development of the corpus callosum. J Neurosci 21:2749–2758
Simi A, Studer M (2018) Developmental genetic programs and activity-dependent mechanisms instruct neocortical area mapping. Curr Opin Neurobiol 53:96–102. https://doi.org/10.1016/j.conb.2018.06.007
Šimić G, Krsnik Ž, Knezović V, Kelović Z, Mathiasen ML, Junaković A, Radoš M, Mulc D, Španić E, Quattrocolo G, Hall VJ, Zaborszky L, Vukšić M, Olucha Bordonau F, Kostović I, Witter MP, Hof PR (2022) Prenatal development of the human entorhinal cortex. J Comp Neurol 530:1–38. https://doi.org/10.1002/cne.25344
Simms ML, Kemper TL, Timbie CM, Bauman ML, Blatt GJ (2009) The anterior cingulate cortex in autism: heterogeneity of qualitative and quantitative cytoarchitectonic features suggests possible subgroups. Acta Neuropathol 118:673–684. https://doi.org/10.1007/s00401-009-0568-2
Sporns O (2011) The human connectome: a complex network. Ann N Y Acad Sci 1224:109–125. https://doi.org/10.1111/j.1749-6632.2010.05888.x
Sporns O, Tononi G, Kötter R (2005) The human connectome: a structural description of the human brain. PLoS Comput Biol 1:0245–0251. https://doi.org/10.1371/journal.pcbi.0010042
Stephan H (1975) Allocortex. In: Stephan H (ed) Handbuch der mikroskopischen Anatomie des Menschen, 1st edn. Springer Verlag, Berlin-Heidelberg, pp 1–971. https://doi.org/10.1016/s0002-9378(31)90375-x
Supèr H, Soriano E, Uylings HBM (1998) The functions of the preplate in development and evolution of the neocortex and hippocampus. Brain Res Rev 27:40–64. https://doi.org/10.1016/S0165-0173(98)00005-8
Thomason ME, Grove LE, Lozon TA, Vila AM, Ye Y, Nye MJ, Manning JH, Pappas A, Hernandez-Andrade E, Yeo L, Mody S, Berman S, Hassan SS, Romero R (2015) Age-related increases in long-range connectivity in fetal functional neural connectivity networks in utero. Dev Cogn Neurosci 11:96–104. https://doi.org/10.1016/j.dcn.2014.09.001
Touroutoglou A, Andreano JM, Adebayo M, Lyons S, Barrett LF (2019) Motivation in the service of allostasis: the role of anterior mid cingulate cortex. Adv Motiv Sci 6:1–25. https://doi.org/10.1016/bs.adms.2018.09.002.Motivation
Triarhou LC (2020) Pre-Brodmann pioneers of cortical cytoarchitectonics II: Carl Hammarberg, Alfred Walter Campbell and Grafton Elliot Smith. Brain Struct Funct 225:2591–2614. https://doi.org/10.1007/s00429-020-02166-8
Utevsky AV, Smith DV, Huettel SA (2014) Precuneus is a functional core of the default-mode network. J Neurosci 34:932–940. https://doi.org/10.1523/JNEUROSCI.4227-13.2014
Utsunomiya H, Yamashita S, Takano K, Okazaki M (2006) Arrangement of fiber tracts forming Probst bundle in complete callosal agenesis: report of two cases with an evaluation by diffusion tensor tractography. Acta Radiol 47:1063–1066. https://doi.org/10.1080/02841850600930025
van den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. J Neurosci 31:15775–15786. https://doi.org/10.1523/JNEUROSCI.3539-11.2011
van den Heuvel MI, Turk E, Manning JH, Hect J, Hernandez-Andrade E, Hassan SS, Romero R, van den Heuvel MP, Thomason ME (2018) Hubs in the human fetal brain network. Dev Cogn Neurosci 30:108–115. https://doi.org/10.1016/j.dcn.2018.02.001
Vasung L, Huang H, Jovanov-Milošević N, Pletikos M, Mori S, Kostović I (2010) Development of axonal pathways in the human fetal fronto-limbic brain: histochemical characterization and diffusion tensor imaging. J Anat 217:400–417. https://doi.org/10.1111/j.1469-7580.2010.01260.x
Vogt BA (1993) Structural organization of cingulate cortex: areas, neurons, and somatodendritic transmitter receptors. In: Vogt BA, Gabriel M (eds) Neurobiology of cingulate cortex and limbic thalamus. Birkhauser, Boston, pp 19–70. https://doi.org/10.1007/978-1-4899-6704-6_2
Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6:533–544. https://doi.org/10.1038/nrn1704.Pain
Vogt BA (2009) Regions and Subregions of the Cingulate Cortex. In: Vogt BA (ed) Cingulate Neurobiology and disease, 1st edn. OUP Oxford, New York, pp 1–31. http://www.cingulumneurosciences.org/uploads/1/1/4/6/114689031/28._chapter_1_vogt.pdf
Vogt BA (2019) Cingulate cortex in the three limbic subsystems. In: Vogt BA (ed) Handbook of clinical neurology, 1st edn. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-444-64196-0.00003-0
Vogt BA, Palomero-Gallagher N (2012) Cingulate cortex. In: Mai J, Paxinos G (eds) The human nervous system, 3rd edn. Elsevier Inc, Amsterdam, pp 943–983
Vogt BA, Pandya DN, Rosene DL (1987) Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol 262:256–270. https://doi.org/10.1002/cne.902620207
Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR (1995) Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol 359:490–506. https://doi.org/10.1002/cne.903590310
Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA et al (2013) Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell 155:997. https://doi.org/10.1016/j.cell.2013.10.020
Yakovlev PI, Locke S, Koskoff DY, Patton RA (1960a) Limbic nuclei of thalamus and connections of limbic cortex I. Organization of the projections of the anterior group of nuclei and of the midline nuclei of the thalamus to the anterior cingulate gyrus and hippocampal rudiment in the monkey. Arch Neurol 3:620–641. https://doi.org/10.1001/archneur.1960.00450060008002
Yakovlev PI, Locke S, Koskoff DY, Patton RA (1960b) Limbic nuclei of thalamus and connections of limbic cortex II. Thalamo-cortical projection of the lateral dorsal nucleus in man. Arch Neurol 3:620–641. https://doi.org/10.1001/archneur.1960.00450060008002
Yakovlev PI, Locke S, Koskoff DY, Patton RA (1960c) Limbic nuclei of thalamus and connections of limbic cortex IV. Thalamocortical projection of the ventral anterior nucleus in man. Arch Neurol 3:620–641. https://doi.org/10.1001/archneur.1960.00450060008002
Yakovlev PI, Locke S, Koskoff DY, Patton RA (1960d) Limbic nuclei of thalamus and connections of limbic cortex V. Thalamocortical projection of the magnocellular medial dorsal nucleus in man. Arch Neurol 3:620–641. https://doi.org/10.1001/archneur.1960.00450060008002
Yücel M, Pantelis C, Stuart GW, Wood SJ, Maruff P, Velakoulis D, Pipingas A, Crowe SF, Tochon-Danguy HJ, Egan GF (2002) Anterior cingulate activation during stroop task performance: a PET to MRI coregistration study of individual patients with schizophrenia. Am J Psychiatry 159:251–254. https://doi.org/10.1176/appi.ajp.159.2.251
Žunić Išasegi I, Radoš M, Krsnik Ž, Radoš M, Benjak V, Kostović I (2018) Interactive histogenesis of axonal strata and proliferative zones in the human fetal cerebral wall. Brain Struct Funct 223:3919–3943. https://doi.org/10.1007/s00429-018-1721-2
Funding
This work was supported by the “Research Cooperability” Program of the Croatian Science Foundation funded by the European Union from the European Social Fund under the Operational Programme Efficient Human Resources 2014–2020 PZS-2019-02-4710 (ZK). This research was also co-funded by the Scientific Centre of Excellence for Basic, Clinical and Translational Neuroscience (project titled “Experimental and clinical research of hypoxic-ischemic damage in perinatal and adult brain” GA KK01.1.1.01.0007 funded by the European Union through the European Regional Development Fund). Some of the fetal material was provided by the Joint MRC/Wellcome Trust grant #099175/Z/12/Z Human Developmental Biology Resource.This work was supported by Croatian Science Fundation project DOK-2020-01-5029 (AJ).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Alisa Junaković, Ivica Kostović, Željka Krsnik and Janja Kopić. The first draft of the manuscript was written by Alisa Junaković and Ivica Kostović and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Conceptualization: IK, AJ, and ŽK; methodology: AJ, JK, and ŽK; figure preparation: AJ, JK, ŽK; writing-original draft preparation: AJ, IK; writing-review and editing: ŽK, AD, PR; supervision, ŽK, IK; project administration, ŽK; funding acquisition, ŽK. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of School of Medicine University of Zagreb (19.11.2015./protocol number 380-59-10106-15-168/314).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Junaković, A., Kopić, J., Duque, A. et al. Laminar dynamics of deep projection neurons and mode of subplate formation are hallmarks of histogenetic subdivisions of the human cingulate cortex before onset of arealization. Brain Struct Funct 228, 613–633 (2023). https://doi.org/10.1007/s00429-022-02606-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-022-02606-7