Abstract
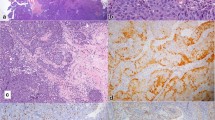
DEK::AFF2 fusion-associated papillary squamous cell carcinoma is a novel entity characterized by its unique translocation and malignant clinical course. In this study, AFF immunohistochemistry (IHC) was performed in recurrent sinonasal papillomas for reviewing the prevalence of undiagnosed DEK::AFF2 carcinomas and to investigate the performance of AFF IHC in diagnosis of DEK::AFF2 carcinomas. Recurrent sinonasal papillomas after surgical excision in a two-decade period were retrieved. Histologic slides were reviewed for features of DEK::AFF2 carcinoma. AFF IHC was performed, and cases with any (> 1%) nuclear positivity were validated by DEK break apart fluorescence in situ hybridization. Totally 43 cases were included, comprising 28 inverted, 6 exophytic, one oncocytic, and 8 non-specified sinonasal papillomas. Five (11.6%) cases exhibited positivity to AFF IHC. Three cases exhibited patchy weak to moderate staining intensity predominantly in a granular cytoplasmic pattern. Two cases exhibited strong and diffuse (> 90%) nuclear staining. Cases showing weak staining were negative for DEK rearrangement, while those with strong staining were positive. Both cases of DEK::AFF2 carcinoma showed aggressive behavior with extensive local invasion and nodal metastasis. Background stromal plasma cells, when present, consistently showed strong and diffuse staining. AFF IHC was further performed in plasmacytoma samples as control and showed strong and diffuse immunoreactivity. A significant minority of recurrent sinonasal papillomas represent DEK::AFF2 carcinomas. Granular, cytoplasmic, or incomplete AFF staining should be considered as negative. In view of the rarity of DEK::AFF2 carcinomas, plasma cells and plasma cell neoplasms are potential for internal and surrogate external controls.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
Elgart K, Faden DL (2020) Sinonasal squamous cell carcinoma: etiology, pathogenesis, and the role of human papilloma virus. Curr Otorhinolaryngol Rep 8(2):111–119
Rampinelli V, Ferrari M, Nicolai P (2018) Intestinal-type adenocarcinoma of the sinonasal tract: an update. Curr Opin Otolaryngol Head Neck Surg 26(2):115–121
Ng JKM, Chan JYK, Li JJX, Tang K, Yeung DCM, Chan ABW (2022) SMARCB1 (INI1)-deficient sinonasal carcinoma with yolk sac differentiation showing co-loss of SMARCA4 immunostaining - a case report and literature review. Head Neck Pathol 16(3):934–941
Taverna C, Agaimy A, Franchi A (2022) Towards a molecular classification of sinonasal carcinomas: clinical implications and opportunities. Cancers 14(6):1463
Riobello C, López-Hernández A, Cabal VN, García-Marín R, Suárez-Fernández L, Sánchez-Fernández P et al (2020) IDH2 mutation analysis in undifferentiated and poorly differentiated sinonasal carcinomas for diagnosis and clinical management. Am J Surg Pathol 44(3):396–405
Edgar M, Caruso AM, Kim E, Foss RD (2017) NUT midline carcinoma of the nasal cavity. Head Neck Pathol 11(3):389–392
Agaimy A, Daum O, Märkl B, Lichtmannegger I, Michal M, Hartmann A (2016) SWI/SNF complex-deficient undifferentiated/rhabdoid carcinomas of the gastrointestinal tract: a series of 13 cases highlighting mutually exclusive loss of SMARCA4 and SMARCA2 and frequent co-inactivation of SMARCB1 and SMARCA2. Am J Surg Pathol 40(4):544–553
Rooper LM, Agaimy A, Dickson BC, Dueber JC, Eberhart CG, Gagan J et al (2021) DEK-AFF2 Carcinoma of the sinonasal region and skull base: detailed clinicopathologic characterization of a distinctive entity. Am J Surg Pathol 45(12):1682–1693
Kuo YJ, Lewis JS Jr, Truong T, Yeh YC, Chernock RD, Zhai C et al (2022) Nuclear expression of AFF2 C-terminus is a sensitive and specific ancillary marker for DEK::AFF2 carcinoma of the sinonasal tract. Mod Pathol 35(11):1587–1595
Bishop JA, Gagan J, Paterson C, McLellan D, Sandison A (2021) Nonkeratinizing squamous cell carcinoma of the sinonasal tract with DEK-AFF2: further solidifying an emerging entity. Am J Surg Pathol 45(5):718–720
Kuo Y-J, Lewis JS, Zhai C, Chen Y-A, Chernock RD, Hsieh M-S et al (2021) DEK-AFF2 fusion-associated papillary squamous cell carcinoma of the sinonasal tract: clinicopathologic characterization of seven cases with deceptively bland morphology. Mod Pathol 34(10):1820–1830
Todorovic E, Truong T, Eskander A, Lin V, Swanson D, Dickson BC et al (2020) Middle ear and temporal bone nonkeratinizing squamous cell carcinomas with DEK-AFF2 fusion: an emerging entity. Am J Surg Pathol 44(9):1244–1250
Ruangritchankul K, Sandison A (2023) DEK::AFF2 fusion carcinomas of head and neck. Adv Anat Pathol 30(2):86–94
Acknowledgements
The authors would like to thank the Core Utilities of Cancer Genomics and Pathobiology (CUHK) for providing the facilities and assistance in support of this research.
Author information
Authors and Affiliations
Contributions
JKMN: conceptualization, investigation, methodology, writing—original draft. CC: investigation, validation, visualization. CT: resources, validation, writing—original draft. AZC: resources. JJXL: conceptualization, data curation, investigation, methodology, visualization, writing—review and editing. ABWC: methodology, investigation, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee and was granted the exemption of requiring written informed consent (2022.531).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ng, J.K.M., Chow, C., Tang, Cy. et al. Prevalence and interpretation of AFF immunostain of DEK::AFF2 fusion-associated papillary squamous cell carcinoma in a retrospective cohort of recurrent sinonasal papillomas. Virchows Arch 484, 119–125 (2024). https://doi.org/10.1007/s00428-023-03717-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03717-0