Abstract
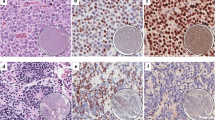
Thymic squamous cell carcinoma (TSC) presents distinct immunohistochemical features with its expression of CD5 and CD117, both of which are rarely expressed in squamous cell carcinoma in other organs. We found insulinoma-associated-1 (INSM1) expression in some TSCs; thus, a series of thymic tumors were examined retrospectively. Using surgically resected thymic tumors (TSC, n = 35; thymic atypical carcinoid [TAC], n = 4; and thymoma, n = 112) and non-neoplastic thymic tissue (n = 26), we evaluated immunohistochemically the expressions of INSM1, ASCL1, SOX2, NE markers (synaptophysin, chromogranin A, and CD56), and conventional TSC markers (CD5 and CD117). INSM1 was expressed in 22 TSCs (63%), whereas the positive frequencies of synaptophysin, chromogranin A, and CD56 were limited to 13, 10, and 1 cases, respectively. The discordance was highly contrasted with concordantly positive TACs. INSM1 and NE makers were rarely expressed in thymomas. INSM1 expression in TSCs was also associated with CD5 expression, which was significantly less frequent in INSM1-negative TSCs. INSM1, ASCL1, and SOX2 expressions were correlated with one another, but none of the single transcription factors or their combinations is associated with NE expression. The non-neoplastic medullary thymic epithelium was dispersedly positive for INSM1, particularly around Hassall’s corpuscles. Despite positive INSM1, a significant decrease in the frequency of NE maker expression may present as a diagnostic pitfall in TSCs. Furthermore, the discordance, which was inherent in the non-neoplastic thymic epithelium, might be a characteristic feature in TSCs.



Similar content being viewed by others
References
Brierley JD, Gospodarowicz MK, Wittekind CH (2017) Inter-national union against cancer TNM classification of malignant tumours, 8th edn. Wiley-Blackwell, Hoboken
WHO Classification of Tumours Editorial Board (2021) Thoracic Tumours, WHO Classification of Tumours, vol 5, 5th edn. International Agency for Research on Cancer, Lyon, pp 81–82
Baine MK, Hsieh MS, Lai WV, Egger JV, Jungbluth AA, Daneshbod Y, Beras A, Spencer R, Lopardo J, Bodd F, Montecalvo J, Sauter JL, Chang JC, Buonocore DJ, Travis WD, Sen T, Poirier JT, Rudin CM, Rekhtman N (2020) SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 15:1823–1835. https://doi.org/10.1016/j.jtho.2020.09.009
Chen C, Breslin MB, Lan MS (2015) INSM1 increases N-myc stability and oncogenesis via a positive-feedback loop in neuroblastoma. Oncotarget 6:36700–36712. https://doi.org/10.18632/oncotarget.5485
Chen C, Notkins AL, Lan MS (2019) Insulinoma-associated-1: from neuroendocrine tumor marker to cancer therapeutics. Mol Cancer Res 17:1597–1604. https://doi.org/10.1158/1541-7786.MCR-19-0286
Dorfman DM, Shahsafaei A, Chan JKC (1997) Thymic carcinomas, but not thymomas and carcinomas of other sites, show CD5 immunoreactivity. Am J Surg Pathol 21:936–940
Fujino K, Motooka Y, Hassan WA, Ali Abdalla MO, Sato Y, Kudoh S, Hasegawa K, Niimori-Kita K, Kobayashi H, Kubota I, Wakimoto J, Suzuki M, Ito T (2015) Insulinoma-associated protein 1 is a crucial regulator of neuroendocrine differentiation in lung cancer. Am J Pathol 185:3164–3177. https://doi.org/10.1016/j.ajpath.2015.08.018
Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, Baron AE, Zeng C, Franklin WA (2003) Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 21:3798–3807. https://doi.org/10.1200/JCO.2003.11.069
Kelly RJ, Petrini I, Rajan A, Wang Y, Giaccone G (2011) Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol 29:4820–4827. https://doi.org/10.1200/JCO.2011.36.0487
Khawaja MR, Nelson RP Jr, Miller N, Badve SS, Loehrer E, Czader M, Perkins SM, Kesler K, Loehrer PJ Sr (2012) Immune-mediated diseases and immunodeficiencies associated with thymic epithelial neoplasms. J Clin Immunol 32:430–437. https://doi.org/10.1007/s10875-011-9644-1
Kuji S, Watanabe R, Sato Y, Iwata T, Hirashima Y, Takekuma M, Ito I, Abe M, Nagashio R, Omae K, Aoki D, Kameya T (2017) A new marker, insulinoma-associated protein 1 (INSM1), for high-grade neuroendocrine carcinoma of the uterine cervix: analysis of 37 cases. Gynecol Oncol 144:384–390. https://doi.org/10.1016/j.ygyno.2016.11.020
Mukhopadhyay S, Dermawan JK, Lanigan CP, Farver CF (2019) Insulinoma-associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: an immunohistochemical study of 345 cases, including 292 whole-tissue sections. Mod Pathol 32:100–109. https://doi.org/10.1038/s41379-018-0122-7
Nonaka D, Henley JD, Chiriboga L, Yee H (2007) Diagnostic utility of thymic epithelial markers CD205 (DEC205) and Foxn1 in thymic epithelial neoplasms. Am J Surg Pathol 31:1038–1044. https://doi.org/10.1097/PAS.0b013e31802b4917
Roden AC, Ahmad U, Cardillo G, Girard N, Jain D, Marom EM, Marx A, Moreira AL, Nicholson AG, Rajan A, Shepherd AF, Simone CB 2nd, Strange CD, Szolkowska M, Truong MT, Rimner A (2022) Thymic carcinomas-a concise multidisciplinary update on recent developments from the thymic carcinoma working group of the International Thymic Malignancy Interest Group. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 17:637–650. https://doi.org/10.1016/j.jtho.2022.01.021
Rooper LM, Bishop JA, Westra WH (2018) INSM1 is a sensitive and specific marker of neuroendocrine differentiation in head and neck tumors. Am J Surg Pathol 42:665–671. https://doi.org/10.1097/PAS.0000000000001037
Rooper LM, Sharma R, Li QK, Illei PB, Westra WH (2017) INSM1 demonstrates superior performance to the individual and combined use of synaptophysin, chromogranin and CD56 for diagnosing neuroendocrine tumors of the thoracic cavity. Am J Surg Pathol 41:1561–1569. https://doi.org/10.1097/PAS.0000000000000916
Rosenbaum JN, Guo Z, Baus RM, Werner H, Rehrauer WM, Lloyd RV (2015) INSM1: a novel immunohistochemical and molecular marker for neuroendocrine and neuroepithelial neoplasms. Am J Clin Pathol 144:579–591. https://doi.org/10.1309/AJCPGZWXXBSNL4VD
Sakane T, Sakamoto Y, Masaki A, Murase T, Okuda K, Nakanishi R, Inagaki H (2021) Mutation profile of thymic carcinoma and thymic neuroendocrine tumor by targeted next-generation sequencing. Clin Lung Cancer 22:92-99 e94. https://doi.org/10.1016/j.cllc.2020.11.010
Schirosi L, Nannini N, Nicoli D, Cavazza A, Valli R, Buti S, Garagnani L, Sartori G, Calabrese F, Marchetti A, Buttitta F, Felicioni L, Migaldi M, Rea F, Di Chiara F, Mengoli MC, Rossi G (2012) Activating c-KIT mutations in a subset of thymic carcinoma and response to different c-KIT inhibitors. Ann Oncol 23:2409–2414. https://doi.org/10.1093/annonc/mdr626
Strobel P, Hartmann E, Rosenwald A, Kalla J, Ott G, Friedel G, Schalke B, Kasahara M, Tomaru U, Marx A (2014) Corticomedullary differentiation and maturational arrest in thymomas. Histopathology 64:557–566. https://doi.org/10.1111/his.12279
Tanigawa M, Nakayama M, Taira T, Hattori S, Mihara Y, Kondo R, Kusano H, Nakamura K, Abe Y, Ishida Y, Okabe Y, Hisaka T, Okuda K, Fujino K, Ito T, Kawahara A, Naito Y, Yamaguchi R, Akiba J, Akagi Y, Yano H (2018) Insulinoma-associated protein 1 (INSM1) is a useful marker for pancreatic neuroendocrine tumor. Med Mol Morphol 51:32–40. https://doi.org/10.1007/s00795-017-0167-6
Tenjin Y, Matsuura K, Kudoh S, Usuki S, Yamada T, Matsuo A, Sato Y, Saito H, Fujino K, Wakimoto J, Ichimura T, Kohrogi H, Sakagami T, Niwa H, Ito T (2020) Distinct transcriptional programs of SOX2 in different types of small cell lung cancers. Lab Invest 100:1575–1588. https://doi.org/10.1038/s41374-020-00479-0
Toriyama A, Mori T, Sekine S, Yoshida A, Hino O, Tsuta K (2014) Utility of PAX8 mouse monoclonal antibody in the diagnosis of thyroid, thymic, pleural and lung tumours: a comparison with polyclonal PAX8 antibody. Histopathology 65:465–472. https://doi.org/10.1111/his.12405
Tsai HK, Hornick JL, Vivero M (2020) INSM1 expression in a subset of thoracic malignancies and small round cell tumors: rare potential pitfalls for small cell carcinoma. Mod Pathol 33:1571–1580. https://doi.org/10.1038/s41379-020-0517-0
Tsuchida M, Umezu H, Hashimoto T, Shinohara H, Koike T, Hosaka Y, Eimoto T, Hayashi JI (2008) Absence of gene mutations in KIT-positive thymic epithelial tumors. Lung Cancer 62:321–325. https://doi.org/10.1016/j.lungcan.2008.03.035
Wang M, Abi-Raad R, Baldassarri R, Adeniran AJ, Cai G (2021) Expression of insulinoma-associated protein 1 in non-small cell lung cancers: a diagnostic pitfall for neuroendocrine tumors. Hum Pathol 115:104–111. https://doi.org/10.1016/j.humpath.2021.06.006
Weissferdt A, Moran CA (2016) Neuroendocrine differentiation in thymic carcinomas: a diagnostic pitfall: an immunohistochemical analysis of 27 cases. Am J Clin Pathol 145:393–400. https://doi.org/10.1093/ajcp/aqv095
Yamada Y, Simon-Keller K, Belharazem-Vitacolonnna D, Bohnenberger H, Kriegsmann M, Kriegsmann K, Hamilton G, Graeter T, Preissler G, Ott G, Roessner ED, Dahmen I, Thomas RK, Strobel P, Marx A (2021) A tuft cell-like signature is highly prevalent in thymic squamous cell carcinoma and delineates new molecular subsets among the major lung cancer histotypes. J Thorac Oncol 16:1003–1016. https://doi.org/10.1016/j.jtho.2021.02.008
Yamada Y, Sugimoto A, Hoki M, Yoshizawa A, Hamaji M, Date H, Haga H, Marx A (2022) POU2F3 beyond thymic carcinomas: expression across the spectrum of thymomas hints to medullary differentiation in type A thymoma. Virchows Archiv : an international journal of pathology 480:843–851. https://doi.org/10.1007/s00428-021-03229-9
Zhang T, Liu WD, Saunee NA, Breslin MB, Lan MS (2009) Zinc finger transcription factor INSM1 interrupts cyclin D1 and CDK4 binding and induces cell cycle arrest. J Biol Chem 284:5574–5581. https://doi.org/10.1074/jbc.M808843200
Funding
This work was supported partly by Grants-in-Aid for Scientific Research (B), JSPS (20H03461), and the National Cancer Center Research and Development Fund (A-3).
Author information
Authors and Affiliations
Contributions
Conceptualization: JK and YY; sample preparation and clinical information: TH, AY, YG, YO, and SW; analysis and investigation: JK and YY; writing—original draft preparation: JK and YY; writing—review and editing: TH, AY, YG, TU, YO, and SW; supervision: TU.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the institutional review board as a part of a larger comprehensive study (NCC IRB No. 2010–077), which are in accordance with the ethical standards of the institutional and/or national research committee and with the declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kashima, J., Hashimoto, T., Yoshida, A. et al. Insulinoma-associated-1 (INSM1) expression in thymic squamous cell carcinoma. Virchows Arch 481, 893–901 (2022). https://doi.org/10.1007/s00428-022-03437-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03437-x