Abstract
Differentiation between penile squamous cell carcinoma patients who can benefit from limited organ-sparing surgery and those at significant risk of lymph node metastasis is based on histopathological prognostic factors including histological grade and tumor histological subtype. We examined levels of interobserver and intraobserver agreement in assessment of histological subtype and grade in 207 patients with penile squamous cell carcinoma. The cases were assessed by seven pathologists from three hospitals located in Sweden and Italy. There was poor to moderate concordance in assessing both histological subtype and grade, with Fleiss kappas of 0.25 (range: 0.02–0.48) and 0.23 (range: 0.07–0.55), respectively. When choosing HPV-associated and non-HPV-associated subtypes, interobserver concordance ranged from poor to good, with a Fleiss kappa value of 0.36 (range: 0.02–0.79). A re-review of the slides by two of the pathologists showed very good intraobserver concordance in assessing histological grade and subtype, with Cohen’s kappa values of 0.94 and 0.91 for grade and 0.95 and 0.84 for subtype. Low interobserver concordance could lead to undertreatment and overtreatment of many patients with penile cancer, and brings into question the utility of tumor histological subtype and tumor grade in determining patient treatment in pT1 tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Penile cancer is a rare malignancy, especially in developed countries. The annual age-standardized global incidence is 0.84 cases per 100,000. However, variations in incidence exist between different countries, likely depending on differences in lifestyle and local practices regarding hygiene, social behavior, and religion [1, 2]. Known risk factors for penile cancer include human papillomavirus (HPV) infection, phimosis, lichen sclerosus and other inflammatory conditions, UVA phototherapy, smoking, and socioeconomic status [3,4,5]. Circumcision has been associated with a reduced risk of penile cancer [4, 6, 7].
A few different histological subtypes of penile squamous cell carcinoma have been described earlier in the literature, but the first report of a clear correlation between certain histological subtypes of invasive penile carcinoma and HPV infection as well as the first histological subclassification was published in 1995 by Gregoire et al. [8]. Approximately 50% of cases of penile cancer are associated with HPV infection [9, 10]. The role of HPV in the carcinogenesis of anogenital tumors and tumors of the head and neck area has been known for a long time. It has been demonstrated that patients with HPV-associated squamous cell carcinoma of the head and neck region have a better prognosis than those with HPV-negative tumors [11,12,13]. Recent studies reported that patients with a HPV-positive penile tumor had better recurrence-free survival rates [9, 14,15,16]. Moreover, tumors that showed an HPV-associated morphology also had lower risk of lymph node metastasis [9]. Multiple histological subtypes of penile squamous cell carcinoma have been added to the classification over the years, and are included in the 2016 World Health Organization (WHO) classification of tumors [17] under two major categories: HPV-related and non-HPV-related subtypes. HPV-associated tumors are usually high grade, most often with basaloid or warty histological subtype. HPV-negative tumors are most often associated with inflammatory conditions such as lichen sclerosus et atrophicus and lichen planus, and usually show a verrucous or usual histological subtype.
The main prognostic factor involved in patient survival is represented by the presence of lymph node metastasis [18]. Approximately 12–25% of patients without clinically palpable lymph nodes will develop occult metastases [19, 20]. Due to the fact that inguinal lymph node dissection (ILND) can be associated with serious complications and increased mortality, predictive histological factors have been used in choosing the best treatment option. It has been shown that tumor histological grade and stage are the most important prognostic elements for prediction of lymph node metastasis in penile cancer patients with clinically negative lymph nodes [19, 21]. In the TNM classification of malignant tumors (TNM), the staging system for penile cancer has been revised multiple times since it was introduced in 1968 [20, 22,23,24]. The histological tumor grade has been included in the latest TNM classification for penile cancer as a criterion for inclusion in the pT1a and pT1b subclasses [24]. According to the WHO and the International Society of Urological Pathology (ISUP), most of the different histological subtypes of penile cancer are associated with certain histological grades and show a different risk for nodal metastasis [17, 25]. The European Association of Urology recommends ILND in primary penile tumors pT1G2, G3 as well as tumors pT2 and pT3. Patients with low-risk tumors (pTa, pTis, or pT1aG1) benefit from organ-sparing surgery and no ILND is needed [26].
Correct identification of histological subtypes that present a higher risk for lymph node metastasis and correct assessment of tumor histological grade are of great importance in management of patients with penile cancer, in order to avoid unnecessary ILND. As penile cancer is a rare type of tumor, most pathologists are not well acquainted with different subtypes and tumor grading. To our knowledge, only a few studies on interobserver reproducibility in assessing the histological tumor grade have been published [27,28,29], and there has been no report on interobserver variation in assessing the histological subtypes of penile cancer.
The aim of this study was to evaluate the levels of interobserver and intraobserver concordance in assessing histological subtype and grade of penile squamous cell cancer, as well as the impact of subjectivity in assessing grade and tumor subtype when choosing treatment management.
Materials and methods
Study cases
We retrospectively reviewed tissue specimens from 229 consecutive patients who underwent surgical treatment for penile cancer between 2009 and 2016 at Örebro University Hospital, Sweden. From these 229 cases, we excluded cases that had limited tumor material (n = 3) as well as cases that included only large glass slides that could not be scanned in our current slide scanner (n = 19). Overall, tissues from a total of 207 cases were included in our study. The study was approved by the Swedish Ethical Review Authority (2019–01923).
Study design
After patient identification in the laboratory informatics system and retrieval of material from the local hospital archive, all the cases were assessed on hematoxylin–eosin stain by a local pathologist. Representative slides from each tumor were chosen and scanned using the Panoramic 250 Flash II system (3DHISTECK, Budapest, Hungary). All the scanned slides were converted into high-resolution digital slides, and the histological evaluation was performed using version 2.1 of the Case Viewer software package (3DHISTECK). The digital slides were evaluated by three experienced pathologists subspecializing in uropathology, a recent specialist, and three residents in pathology from three hospitals in Sweden and Italy. Two of the pathologists (graders 1 and 7) are subspecialized in the diagnosis of penile cancer, and work in hospitals specializing in the treatment of penile cancer. The following variables were assessed: tumor histological subtype and tumor histological grade. All pathologists followed the histological criteria recommended by WHO classification of tumors [17] and its guidelines for grading of penile cancer. Information regarding the presence/absence of HPV in the tumor material was unknown to the pathologists, in order to avoid subjective assessment of the histological tumor subtype. Two of the most experienced pathologists (graders 1 and 2) also re-examined the digital slides a month after the first evaluation in order to assess the level of intraobserver agreement.
Tumor histological subtype
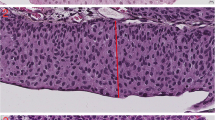
The histological subtype of primary penile tumors (Fig. 1) was assessed according to criteria published by the WHO classification of tumors of the urinary system and ISUP recommendation (2016) [17, 25]. According to this classification, penile squamous cell carcinoma is subclassified in twelve different histological subtypes divided into two categories. Non-HPV-related carcinomas include the following subtypes: usual, verrucous with its variants, carcinoma cuniculatum and pseudohyperplastic, pseudoglandular, papillary, adenosquamous, sarcomatoid as well as mixed forms, most often hybrid verrucous carcinomas. The HPV-related carcinoma category includes basaloid, warty, clear cell, lymphoepithelioma-like carcinoma, and mixed (most often warty-basaloid) subtypes.
Histological grade
The tumors were graded using a three-tiered system based on ISUP/WHO recommendations [17, 25]. In this system, grade 1 tumors are well differentiated with minimal cell atypia, and grade 3 tumors show no maturation and have a high cell pleomorphism and high mitotic activity. Tumors that do not meet the criteria for grades 1 or 3 belong to grade 2 (Fig. 2). Squamous cell carcinomas are usually heterogeneous and show different grades in different parts of the tumor; the histological grade is assigned based on the highest observed grade, as any proportion of high grade is relevant.
Statistical analysis
Interobserver and intraobserver concordance were assessed by calculating Cohen’s kappa (κ) between pairs of observers for histological grade, subtype, and inclusion in the HPV-positive or HPV-negative group. Cohen’s κ was calculated using version 25 of IBM SPSS Statistics (IBM Corp., Armonk, NY, USA), and Fleiss’ κ for overall concordance among all seven graders was calculated in R [30], using the package irr [31]. The degree of concordance was classified as poor at κ values of 0.00–0.20, fair at κ values of 0.21–0.40, moderate at κ values of 0.41–0.60, good at κ values of 0.61–0.80, and very good at κ values of 0.81–1.00 [32].
Results
Histological subtype
The level of concordance in assessing tumor histological subtype was fair (Table 1, upper right), with a Fleiss’ κ of 0.25 (Cohen’s κ values between 0.02 and 0.48). All twelve histological subtypes were identified, as well as eleven mixed forms (n = 23).
When analyzing HPV-associated and non-HPV-associated subtypes (n = 2) (Table 1, lower left), the interobserver concordance was fair, with a Fleiss’ κ value of 0.36 (Cohen’s κ values between 0.02 and 0.79). Tumors with mixed morphology that included both HPV-associated and non-HPV-associated subtypes were included in the HPV-associated tumor group. The histological subtypes with best interobserver concordance using Fleiss’ κ were basaloid, sarcomatoid, lymphoepithelioma-like, and usual (κ values of 0.51, 0.50, 0.83, and 0.33, respectively). For mixed histological subtypes, there was poor to fair concordance, with Fleiss’ κ values ranging from − 0.001 to 0.036. The best concordance in assessing tumor histological subtype in general was seen between the pathologists specializing in diagnosis of penile tumors (graders 1 and 7).
Graders 1 and 2 also re-reviewed the scanned slides in order to assess the intraobserver agreement on histological subtype. There was a very good concordance between the first review and the re-review, with Cohen’s κ values of 0.95 and 0.81, respectively, for the two graders (intraobserver agreement of 96.6% and 88.4%).
Histological grade
The interobserver agreement in histological tumor grade (Table 2) showed a fair concordance, with a Fleiss’ κ of 0.23 (Cohen’s κ values between 0.07 and 0.55). The highest level of concordance for grade was found between the most experienced pathologists.
The intraobserver agreement between graders 1 and 2 in assessing tumor grade was 94.2% and 96.1%, respectively. The concordance was very good, with Cohen’s κ values of 0.94 and 0.91.
When assessing grade, the different pathologists graded 2.4–34.3% of the evaluated tumors as G1 tumors, 21.3–70.0% as G2 tumors, and 27.5–62.8% as G3 tumors. Three of the seven pathologists graded more than 50% of the tumors as grade 2, while two graders (4 and 5) graded more than 50% of the tumors as grade 3. The last grader (grader 2) had an equal distribution of grade 1–3 tumors (Table 3).
Discussion
When it comes to survival of patients with penile cancer, the presence of inguinal lymph node metastasis is one of the most important prognostic factors. Up to 25% of patients with penile cancer will benefit from ILND. At the same time, the procedure is associated with short- or long-term complications like wound dehiscence, necrosis, infection, lymphedema, and lymphocele in up to 84% of cases [33]. Thus, the identification of patients whose tumors are low or high risk for lymph node metastasis is crucial, in order to avoid both undertreatment and overtreatment.
Previous studies have shown an association between tumor histological subtype and the risk of lymph node metastasis in penile cancer [9, 34,35,36,37]. While the verrucous variant of squamous cell carcinoma and its variants pseudohyperplastic and carcinoma cuniculatum have a very low potential for metastasis and an excellent prognosis, the sarcomatous and basaloid subtypes have been associated with an increased risk of lymph node metastasis and mortality [17, 34, 36]. The European Association of Urology guidelines classify the different subtypes of penile cancer into three risk groups based on the risk of lymph node metastasis. The low-risk group consists of the verrucous subtype and its variants, along with the warty and papillary subtypes. The usual and mixed subtypes are included in the intermediate-risk group, and the high-risk group includes basaloid, adeno-squamous, sarcomatoid, and poorly differentiated variant of warty subtypes [26]. In the light of these data, correct identification of the histological subtype plays an important role in assessing the risk of nodal metastasis and in choosing the best treatment. While our data show the best interobserver concordance regarding usual, basaloid, lymphoepithelioma-like, and sarcomatoid subtypes of squamous cell cancer, poor concordance was observed in the mixed subtypes that represent up to 25% of penile tumors [17] and which are included in the intermediate-risk group.
Most of the pathologists participating in this study had a tendency to choose pure subtypes instead of mixed variants. This can have an important impact on the choice of treatment. While verrucous cancer, a low-risk tumor, can in certain cases have a more limited treatment approach with organ-sparing surgery and surveillance of lymph node status, the verrucous-usual mixed subtype is an intermediate-risk tumor which needs a more aggressive local surgical approach and can benefit from dynamic sentinel node biopsy or modified ILND. Our finding of higher levels of interobserver concordance between more experienced pathologists shows the importance of good knowledge of uropathology and good experience as a pathologist in diagnosis of penile tumors. The ability to recognize different histological subtypes included in the different risk groups, which have different treatment approaches, is of great importance [26]. The pathologist should be able to identify the correct subtype and should include it in the pathology report.
Higher survival rates in patients with HPV-related penile cancer, along with the future possibility of different treatment options based on the histological subtype, make a correct assessment of the histological HPV-associated and non-HPV-associated subtypes even more important. Because HPV analysis is not available in many countries with high incidence of penile cancer, a good knowledge of morphological diagnostic criteria is needed. Most of the histological subtypes can be recognized on the basis of characteristic morphological features with no need for HPV analysis, but in difficult cases the WHO recommends the use of p16 immunostaining [25]. Our study shows that pathologists who have experience in working with penile cancer have a good concordance in identifying HPV-related and non-HPV-related histological subtypes of squamous cell carcinoma. Further studies of the histological tumor characteristics and the role of HPV infection in carcinogenesis might lead to better and more objective prognostic factors for patients with penile cancer.
The latest TNM classification from 2016 includes tumor histological grade in staging of penile cancer. Thus, stage pT1 has been divided into pT1a (histological grade G1–G2 without vascular invasion) and pT1b (histological G3 and/or vascular invasion). According to Solsona et al. [19], there are three risk group categories of penile cancer. The low-risk group comprises tumors at stage pT1/G1; the intermediate-risk group includes tumors pT1, G2–3 and pT2, G1; and the high-risk group consists of tumors pT2, G2–G3 and pT3, pT4 [19]. Patients in the high-risk and intermediate-risk groups with clinically negative inguinal lymph nodes can benefit from ILND as part of standard treatment. Tumor stage and histological grade also play an important role in treatment management of the primary tumor, with possibilities of different techniques of organ-sparing surgery for tumors pT1a and pT1b [38]. This is especially important in younger patients who are still sexually active, and in whom an organ-sparing surgical treatment with good cosmetic and functional results has an important impact on quality of life.
Our study showed poor to moderate concordance between different pathologists in assessing the histological grade, with a higher level of concordance between experienced pathologists. The broad intervals in percentages of G1, G2, and G3 tumors in individual assessments raises the question of whether risk group categories and the division of pT1 stage should be based on this subjective prognostic factor. For example, in terms of grade distribution (Table 3), G1 tumors represented between 2.4 and 34.3% of the total cases depending on the assessing pathologist. If all the cases were stage pT1, between 5 and 71 patients (total n = 207) would benefit from organ-sparing surgery and no ILND would be needed. The question remains of how many of these patients will be undertreated or overtreated given such large variation in the results of histological grading. Histological grading of squamous cell carcinoma lacks a standard grading system, and is highly subjective [27,28,29, 39]. Studies of interobserver agreement on histological grading of squamous cancer in general have been performed using different grading systems, but all have shown low levels of agreement [27, 28, 39,40,41]. Among all the different TNM classifications of squamous cell carcinoma in specific locations, only penile cancer has histological grade as part of TNM classification.
The different levels of experience of the reviewers in this study can be seen as a limitation. On the other hand, our study has been able to reveal the importance of both subspecialization in uropathology and experience in assessing grade and tumor subtype when it comes to penile pathology. The major strengths are the large number of cases included and the substantial number of assessing pathologists.
Conclusions
Our study demonstrates a broad spectrum of interobserver concordance between different pathologists in assessing the histological tumor subtype, in choosing HPV-associated and HPV-negative subtypes of squamous cell carcinoma, and in histological grading of penile cancer. The level of disagreement depends on personal subjectivity, the lack of standardized objective morphologic grading criteria, and level of experience in the diagnosis of penile cancer. The use of tumor histological subtype as a criterion for separation into different risk groups and the division of pT1 stage based on histological grade might not have the best prognostic value when it comes to choosing the right treatment option. Most pathologists rarely have the chance to see cases of penile cancer in daily practice and are not aware of the new classifications and recommendations, and thus lack experience in grading and subtyping such tumors; this, in turn, has an impact on patient management. The higher level of concordance found between the experienced pathologists in this study shows the importance of good knowledge of penile pathology, and of pathology in general, in order to avoid overtreatment or undertreatment of patients with penile cancer. The subspecialty of pathologists surely improves the concordance and the overall quality of the diagnoses in penile pathology. Further studies of tumor biology and etiological factors are needed in order to discover more reliable prognostic factors and for improvement of the diagnosis and treatment of patients with penile cancer.
Abbreviations
- HPV:
-
Human papillomavirus
- ISUP:
-
International Society of Urological Pathology
- WHO:
-
World Health Organization
- G1–3:
-
Histological grade
- TNM:
-
TNM classification of malignant tumors
- ILND:
-
Inguinal lymph node dissection
References
Montes Cardona CE, García-Perdomo HA (2017) Incidence of penile cancer worldwide: systematic review and meta-analysis. Rev Panam Salud Publica 41:e117
Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, Giuliano AR (2007) Incidence trends in primary malignant penile cancer. Urol Oncol 25(5):361–367. https://doi.org/10.1016/j.urolonc.2006.08.029
Douglawi A, Masterson TA (2017) Updates on the epidemiology and risk factors for penile cancer. Transl Androl Urol 6(5):785–790. https://doi.org/10.21037/tau.2017.05.19
Daling JR, Madeleine MM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, Carter JJ, Porter PL, Galloway DA, McDougall JK, Krieger JN (2005) Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer 116(4):606–616. https://doi.org/10.1002/ijc.21009
D’Hauwers KW, Depuydt CE, Bogers JJ, Noel JC, Delvenne P, Marbaix E, Donders AR, Tjalma WA (2012) Human papillomavirus, lichen sclerosus and penile cancer: a study in Belgium. Vaccine 30(46):6573–6577. https://doi.org/10.1016/j.vaccine.2012.08.034
Maden C, Sherman KJ, Beckmann AM, Hislop TG, Teh CZ, Ashley RL, Daling JR (1993) History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst 85(1):19–24. https://doi.org/10.1093/jnci/85.1.19
Larke NL, Thomas SL, dos Santos SI, Weiss HA (2011) Male circumcision and penile cancer: a systematic review and meta-analysis. Cancer Causes Control 22(8):1097–1110. https://doi.org/10.1007/s10552-011-9785-9
Gregoire L, Cubilla AL, Reuter VE, Haas GP, Lancaster WD (1995) Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst 87(22):1705–1709. https://doi.org/10.1093/jnci/87.22.1705
Eich ML, Del Carmen Rodriguez Pena M, Schwartz L, Granada CP, Rais-Bahrami S, Giannico G, Amador BM, Matoso A, Gordetsky JB (2020) Morphology, p16, HPV, and outcomes in squamous cell carcinoma of the penis: a multi-institutional study. Hum Pathol 96(79):86. https://doi.org/10.1016/j.humpath.2019.09.013
Olesen TB, Sand FL, Rasmussen CL, Albieri V, Toft BG, Norrild B, Munk C, Kjær SK (2019) Prevalence of human papillomavirus DNA and p16(INK4a) in penile cancer and penile intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol 20(1):145–158. https://doi.org/10.1016/s1470-2045(18)30682-x
O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA (2012) Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol 48(12):1191–1201. https://doi.org/10.1016/j.oraloncology.2012.06.019
Kaplon AW, Galloway TJ, Bhayani MK, Liu JC (2020) Effect of HPV status on survival of oropharynx cancer with distant metastasis. Otolaryngol Head Neck Surg 163(2):372–374. https://doi.org/10.1177/0194599820913604
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363(1):24–35. https://doi.org/10.1056/NEJMoa0912217
Bethune G, Campbell J, Rocker A, Bell D, Rendon R, Merrimen J (2012) Clinical and pathologic factors of prognostic significance in penile squamous cell carcinoma in a North American population. Urology 79(5):1092–1097. https://doi.org/10.1016/j.urology.2011.12.048
Sand FL, Rasmussen CL, Frederiksen MH, Andersen KK, Kjaer SK (2018) Prognostic significance of HPV and p16 status in men diagnosed with penile cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 27(10):1123–1132. https://doi.org/10.1158/1055-9965.Epi-18-0322
Lont AP, Kroon BK, Horenblas S, Gallee MP, Berkhof J, Meijer CJ, Snijders PJ (2006) Presence of high-risk human papillomavirus DNA in penile carcinoma predicts favorable outcome in survival. Int J Cancer 119(5):1078–1081. https://doi.org/10.1002/ijc.21961
Moch H, Humphrey PA, Ulbright TM, Reuter VE (2016) WHO classification of tumours of the urinary system and male genital organs, vol 8. International Agency for Research on Cancer (IARC)
Ficarra V, Akduman B, Bouchot O, Palou J, Tobias-Machado M (2010) Prognostic factors in penile cancer. Urology 76(2 Suppl 1):S66–S73. https://doi.org/10.1016/j.urology.2010.04.008
Solsona E, Iborra I, Rubio J, Casanova JL, Ricós JV, Calabuig C (2001) Prospective validation of the association of local tumor stage and grade as a predictive factor for occult lymph node micrometastasis in patients with penile carcinoma and clinically negative inguinal lymph nodes. J Urol 165(5):1506–1509. https://doi.org/10.1016/S0022-5347(05)66337-9
Leijte JA, Gallee M, Antonini N, Horenblas S (2008) Evaluation of current TNM classification of penile carcinoma. J Urol 180(3):933–938. https://doi.org/10.1016/j.juro.2008.05.011
Aita GA, de Cássio ZS, da Costa WH, Guimarães GC, Soares FA, Giuliangelis TS (2016) Tumor histologic grade is the most important prognostic factor in patients with penile cancer and clinically negative lymph nodes not submitted to regional lymphadenectomy. Int Braz J Urol 42(6):1136–1143. https://doi.org/10.1590/S1677-5538.IBJU.2015.0416
Leijte JA, Horenblas S (2009) Shortcomings of the current TNM classification for penile carcinoma: time for a change? World J Urol 27(2):151–154. https://doi.org/10.1007/s00345-008-0308-6
Heyns CF, Mendoza-Valdés A, Pompeo AC (2010) Diagnosis and staging of penile cancer. Urology 76(2 Suppl 1):S15–S23. https://doi.org/10.1016/j.urology.2010.03.002
Brierley JD, Gospodarowicz MK, Wittekind C (2017) TNM classification of malignant tumours. John Wiley & Sons
Cubilla AL, Velazquez EF, Amin MB, Epstein J, Berney DM, Corbishley CM (2018) The World Health Organisation 2016 classification of penile carcinomas: a review and update from the International Society of Urological Pathology expert-driven recommendations. Histopathology 72(6):893–904. https://doi.org/10.1111/his.13429
Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N (2015) EAU guidelines on penile cancer: 2014 update. Eur Urol 67(1):142–150. https://doi.org/10.1016/j.eururo.2014.10.017
Gunia S, Burger M, Hakenberg OW, May D, Koch S, Jain A, Birnkammer K, Wieland WF, Otto W, Hofstädter F, Fritsche HM, Denzinger S, Gilfrich C, Brookman-May S, May M (2013) Inherent grading characteristics of individual pathologists contribute to clinically and prognostically relevant interobserver discordance concerning Broders’ grading of penile squamous cell carcinomas. Urol Int 90(2):207–213. https://doi.org/10.1159/000342639
Kakies C, Lopez-Beltran A, Comperat E, Erbersdobler A, Grobholz R, Hakenberg OW, Hartmann A, Horn LC, Höhn AK, Köllermann J, Kristiansen G, Montironi R, Scarpelli M, Protzel C (2014) Reproducibility of histopathologic tumor grading in penile cancer—results of a European project. Virchows Arch 464(4):453–461. https://doi.org/10.1007/s00428-014-1548-z
Naumann CM, Alkatout I, Hamann MF, Al-Najar A, Hegele A, Korda JB, Bolenz C, Klöppel G, Jünemann KP, van der Horst C (2009) Interobserver variation in grading and staging of squamous cell carcinoma of the penis in relation to the clinical outcome. BJU Int 103(12):1660–1665. https://doi.org/10.1111/j.1464-410X.2009.08362.x
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
Gamer M, Lemon J, Fellows I, Singh P (2019) Various coefficients of interrater reliability and agreement. https://CRAN.R-project.org/package=irr
Altman DG (1990) Practical statistics for medical research. CRC Press
Horenblas S (2001) Lymphadenectomy for squamous cell carcinoma of the penis. Part 2: the role and technique of lymph node dissection. BJU Int 88(5):473–483. https://doi.org/10.1046/j.1464-410x.2001.00379.x
Cubilla AL, Reuter V, Velazquez E, Piris A, Saito S, Young RH (2001) Histologic classification of penile carcinoma and its relation to outcome in 61 patients with primary resection. Int J Surg Pathol 9(2):111–120. https://doi.org/10.1177/106689690100900204
Sanchez DF, Soares F, Alvarado-Cabrero I, Cañete S, Fernández-Nestosa MJ, Rodríguez IM, Barreto J, Cubilla AL (2015) Pathological factors, behavior, and histological prognostic risk groups in subtypes of penile squamous cell carcinomas (SCC). Semin Diagn Pathol 32(3):222–231. https://doi.org/10.1053/j.semdp.2014.12.017
Wang JY, Gao MZ, Yu DX, Xie DD, Wang Y, Bi LK, Zhang T, Ding DM (2018) Histological subtype is a significant predictor for inguinal lymph node metastasis in patients with penile squamous cell carcinoma. Asian J Androl 20(3):265–269. https://doi.org/10.4103/aja.aja_60_17
Dai B, Ye DW, Kong YY, Yao XD, Zhang HL, Shen YJ (2006) Predicting regional lymph node metastasis in Chinese patients with penile squamous cell carcinoma: the role of histopathological classification, tumor stage and depth of invasion. J Urol 176(4 Pt 1):1431–1435. https://doi.org/10.1016/j.juro.2006.06.024
Regionala Cancercentrum (2019) Nationellt vårdprogram peniscancer. 12 May 2019. https://kunskapsbanken.cancercentrum.se/diagnoser/peniscancer/
Bryne M, Nielsen K, Koppang HS, Dabelsteen E (1991) Reproducibility of two malignancy grading systems with reportedly prognostic value for oral cancer patients. J Oral Pathol Med 20(8):369–372. https://doi.org/10.1111/j.1600-0714.1991.tb00946.x
Slaton JW, Morgenstern N, Levy DA, Santos MW Jr, Tamboli P, Ro JY, Ayala AG, Pettaway CA (2001) Tumor stage, vascular invasion and the percentage of poorly differentiated cancer: independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J Urol 165(4):1138–1142
Chaux A, Torres J, Pfannl R, Barreto J, Rodriguez I, Velazquez EF, Cubilla AL (2009) Histologic grade in penile squamous cell carcinoma: visual estimation versus digital measurement of proportions of grades, adverse prognosis with any proportion of grade 3 and correlation of a Gleason-like system with nodal metastasis. Am J Surg Pathol 33(7):1042–1048. https://doi.org/10.1097/PAS.0b013e31819aa4c9
Funding
Open access funding provided by Örebro University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by Luiza Dorofte. Data collection was performed by Luiza Dorofte, Diane Grélaud, Michelangelo Fiorentino, Francesca Giunchi, Costantino Ricci, Tania Franceschini, and Mattia Riefolo. Analysis was performed by Luiza Dorofte, Sabina Davidsson, Jessica Carlsson, Gabriella Lillsunde Larsson, and Mats G. Karlsson. The first draft of the manuscript was written by Luiza Dorofte and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Swedish Ethical Review Authority (2019–01923).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dorofte, L., Grélaud, D., Fiorentino, M. et al. Low level of interobserver concordance in assessing histological subtype and tumor grade in patients with penile cancer may impair patient care. Virchows Arch 480, 879–886 (2022). https://doi.org/10.1007/s00428-021-03249-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03249-5