Abstract
Liver biopsy is crucial for the diagnosis of autoimmune hepatitis (AIH), and new reproducible histological criteria would be highly desirable, especially in acute-on-chronic cases. The aims of the present study were (i) to evaluate the AIH histopathological criteria as a function of the time and modality of AIH onset, and (ii) to validate the count of apoptotic bodies in the portal tracts as a histopathological criterion for AIH diagnosis. Sixty-five patients were retrospectively enrolled: 20 underwent biopsy for the first diagnosis and 45 had a previous histological AIH diagnosis. Biopsies were revised, and all histological variables were collected, including the lymphocytic apoptotic bodies in the portal tracts. Clinical and serological data were revised as well. First-diagnosis patients showed a higher grade of inflammation (p = 0.001), but also worse portal fibrosis (p = 0.001). The apoptotic body count was higher in first-diagnosis patients than in follow-up patients (p = 0.002), and it was strongly correlated to inflammation. Using the apoptotic body count among the simplified AIH score variables, the first-biopsy patients in the “definite” category rose from 42 to 68%. Our results confirm the histopathological criteria proposed by the literature and introduce the count of portal apoptotic bodies for the diagnosis of active AIH, especially in first biopsies without other classic features, as well as in AIH diagnostic score, albeit future studies are required to find a definite cutoff.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoimmune hepatitis (AIH) is a liver immune-mediated inflammatory disease. A recent meta-analysis indicated an incidence of 1.37 cases per 100,000 population and a prevalence of 17.44 cases per 100,000 population although real data could be different [1]. Women/men ratio is 3.6/1 and any age can be affected [2, 3]. Pathogenesis of AIH remains unknown, although the most probable cause is likely to be represented by an altered lymphocytic self-tolerance towards liver antigens: accordingly, AIH develops in subjects with a genetic predisposition, in particular as far as specific allelic HLA haplotypes are concerned [4].
Diagnosis of AIH is based upon clinical, serological, and histological data. These features were at first included in a scoring system in 1992 [5], reviewed in 1998 by the International Autoimmune Hepatitis Group (IAIHG) [6], and finally simplified in 2008 [7]. Clinical presentation of the disease is variable, ranging from liver enzyme alterations to fulminant hepatitis characterized by massive hepatic necrosis. However, the clinical presentation is usually insidious and characterized by fatigue, nausea, anorexia, abdominal pain, weight loss, and arthralgia [8, 9]. Serological levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and gamma-glutamyltransferase (GGT) are increased, and the levels of serum IgG might be elevated, usually 1.2–3-time normal range. Diagnostic serum antibodies include antinuclear antibody (ANA), anti-smooth muscle antibody (SMA), anti-liver kidney microsomal antibody (LKM-1) [10, 11]. The different autoantibody pattern allows for differentiating between autoimmune hepatitis type 1 (AIH-1, elevated ANA/SMA) and autoimmune hepatitis type 2 (AIH-2, elevated LKM-1 and/or anti-liver cytosol antigen type 1, LC-1). Histology on needle biopsy is necessary for a definite diagnosis of AIH, and it should be performed in patients with acute or chronic liver disease with unknown origin, particularly in the context of hypergammaglobulinemia, with specific autoantibody profile and with the exclusion of other causes of acute and chronic liver diseases [12]. The key role of biopsy is its adequacy and promptness. It results in being a crucial therapy to delay the progression of liver fibrosis. The European Association for the Study of the Liver (EASL) defined the criteria for the histopathological diagnosis of AIH [12], introducing the categories “definite,” “probable,” and “exclusion.” The main histopathological features include interface hepatitis with dense lymphocytic infiltrate with a variable amount of plasma cells, hepatocellular rosette formation, emperipolesis, and hepatocyte swelling and/or pyknotic necrosis are the typical hallmarks of AIH [7, 13]. However, these criteria have never been standardized among different centers [12]. The presence of plasma cells is not specific either, since they can be present also in viral hepatitis (such as HBV and HCV). The role of plasma cells in AIH is not clear, and a numerical cutoff for plasma cells does not exist [13].
Recently, Balitzer et al. proposed a new histological score based on the grade of necro-inflammatory activity, combining the Ishak activity grade and the number of plasma cells; moreover, they proposed the use of Keratin 7 (K7) and/or copper stain to detect histological signs of chronic cholestasis which would be useful to exclude a biliary disease. The utility of such a diagnostic system in clinical practice has not been assessed so far [14]. Other authors observed that, beyond the cytonecrotic damage, regenerative changes like hepatocytic rosettes and cobblestone appearance of the laminae could be used as AIH criteria, representing an indirect sign of cytonecrosis [15, 16].
In this scenario, new histological criteria, which could cost-effectively fortify the diagnosis of AIH, would be highly desirable.
In our routine practice, we noticed that apoptotic lymphocytes are common in portal tracts in AIH, but lymphocyte apoptosis in the human liver with AIH has never been systematically studied so far.
Apoptosis is a non-inflammatory programmed cell death (PCD) usually triggered by irreversible cell damage. Apoptosis of inflammatory and epithelial cells has been already described in autoimmune diseases, such as systemic lupus erythematosus (SLE) and bowel transplant rejection [17, 18], indicating that a link between disorders of the self-tolerance and apoptosis exists.
The aims of the present study were (i) to evaluate the histopathological criteria proposed by the literature for AIH diagnosis in a cohort of patients with ascertained AIH who come to a tertiary referral center (TRC), as a function of the time and modality of AIH onset; (ii) to systematically investigate lymphocyte apoptosis in a series of well-characterized AIH. Moreover, we analyzed how the new recently proposed score for the histological diagnosis of AIH fits with our series.
Materials and methods
Study population, clinical data, and biopsy timing
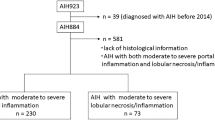
All patients with proved AIH who underwent liver biopsy for the first time in our TRC from 2010 to 2017 were retrospectively enrolled. Inclusion criteria were age ≥ 18 years, execution of the liver biopsy at admission in our center. Exclusion criteria were overlap between AIH and any other liver diseases, IgG4-related disease, and proven or highly suspected drug-induced liver injury (DILI).
Sixty-five patients satisfied the criteria of enrollment, 44 (67.7%) females and 21 (32.3%) males, mean age at biopsy 49.4 ± 15.8 years (range 19–79 years). In 20 (30.8%) cases, the admission to the TRC occurred for a clinical suspect of AIH (virus-negative acute hepatitis), and the first biopsy confirmed the diagnosis; this group defines as first-biopsy patient (FBP) group. Forty-five patients (69.2%) enrolled with a previous histological diagnosis of AIH made in other centers; in these patients, defined as follow-up patient (FUP) group, the admission for follow-up and revaluation (N = 32) or acute flare (N = 13) was made. For FUP, the mean time from the first diagnosis of AIH to the enrollment in TRC was 90.4 ± 54.9 months (range 1–192 months).
Since drug-induced liver damage represents a significant differential diagnosis with AIH, we excluded a priori those cases with suspect DILI: as a result, in our series, a history of intake of non-steroidal anti-inflammatory drugs was recorded in 7 (10.1%) cases, 5 FUP and 2 FBP. All patients of the FUP group received corticosteroid therapy, with 29 relapse cases registered over time: these cases were treated with steroids (n = 14), steroids plus azathioprine (n = 10), azathioprine alone (n = 4), and cyclosporin (n = 1).
Limited to the study of lymphocyte apoptosis and retrospectively selected from our archive, liver biopsies from 25 HCV-positive hepatitis (10 females and 15 males, mean age 51.6 ± 19.2 years, range 27–71), from 10 patients with primary biliary cholangitis (PBC; all women, mean age 50.3 ± 15.6, range 42–66), and 10 patients with DILI (6 females and 4 males, mean age 55.9 ± 21.1 years, range 20–83) function as control groups.
The clinical variables, including AST, ALT, GGT, ALP, conjugated, unconjugated, total bilirubin, and total gamma globulin, were collected during admission/biopsy. The occurrence and titers of the SMA, ANA, LKM, and AMA autoantibodies, further the time and modality of AIH onset, were also recorded. A summary of raw clinical data is shown in Table 1.
Histopathology and immunohistochemistry
All liver biopsies were percutaneously obtained, formalin-fixed, paraffin-embedded, routinely processed, and stained with hematoxylin–eosin (HE) and reticulin. The biopsies of all patients were revised by two pathologists (one trainee pathologist and one dedicated pathologist) at a multiple-head microscope.
Portal inflammation, interface hepatitis, lobular inflammation/necrosis, and fibrosis stage were assessed by Ishak’s score [19]. Plasma cells were evaluated as an absolute number in 3 “hot spots” at higher magnification. We obtained the AIH score proposed by Balitzer et al. [14], combining necro-inflammatory activity according to Ishak and the number of plasma cells. Score 0 was assigned in cases without features of AIH (or with chronic cholestasis utilizing K7 immunoreactivity and rhodanine stain for copper deposits), score 1 in cases of hepatitis with mild-to-moderate activity (Ishak’s A2-B1-C2) and with negativity for K7 and rhodanine stain, and score 2 in cases with numerous plasma cells (eventually in clusters) and/or hepatitis with severe activity (Ishak’s A3-B2-C3) [14].
Besides, the presence of emperipolesis, hepatocytic rosettes, and/or “cobblestone” appearance were recorded, as the presence of bile duct changes, which was, according to the literature, defined as irregularity in shape and/or size of bile ducts [20].
Rhodanine stain and K7 IHC (clone SP52, prediluted, BenchMark ULTRA® immunostainer, Ventana Medical Systems) were performed. Based on granular cytoplasmic staining in periportal hepatocytes, rhodanine stains suggest an ambivalence as negative or positive. If at least three periportal hepatocytes were positive in at least two foci, stains of K7 were logged as expressed in hepatocytes; all the other cases were scored as negative [14].
In all cases based on clinical and histological variables, the simplified AIH score was evaluated according to the original description [7].
Lymphocytic apoptotic bodies were defined as round, small, basophilic, globule devoid of cytoplasm within lymphocytes infiltration. Apoptotic bodies were counted in the portal tracts in at least 3 “hot spots” at high magnification and therefore reported as mean for each case.
Statistical analysis
All statistical analyses were carried out employing SPSS® software for Windows.
Continuous variables are reported as mean ± standard deviation and range; discrete variables are reported as frequency and percentage. Chi-square test, ANOVA test, Mann–Whitney test, t-test, and Spearman’s test were applied as appropriate. A correlation coefficient p < 0.05 was considered statistically significant.
Results
Histopathological differences between follow-up patients and first-biopsy patients
Portal inflammation according to Ishak was absent in 13 (20.0%) cases, grade 1 in 20 (30.8%), grade 2 in 23 (35.4%) and grade 3 in 9 (13.8%). Interface hepatitis according to Ishak was absent in 16 (24.6%) cases, grade 1 in 14 (21.5%), grade 2 in 20 (30.8%), grades 3 and 4 in 15 (23.1%). Lobular necrosis/inflammation was absent in 2 (3.1%) cases, grade 1 in 43 (66.2%), grade 2 in 12 (18.5%), and grade 3 in 8 (12.3%), with Councilman’s bodies in 18 (27.7%) cases. Mean plasma cells count was 16.5 ± 14.2/hot spot (range 0–50). Emperipolesis was observed in 13 (20.0%) cases, hepatocytic rosettes in 57 (87.7%) and “cobblestone” appearance in 24 (36.9%). Bile ducts were normal in 25 (38.5%) cases: in 11 (16.9%) cases, focal bile duct alterations were present, and in 29 (44.6%) cases, diffuse bile duct alterations were present. Stage was mild (stage 0–2 according to Ishak) in 47 (72.3%) biopsies, stage 3 was recorded in 12 (18.4%), stage 4 in 2 (3.1%), and stage 5 (pre-cirrhotic stage) in 4 (6.2%).
The score for AIH diagnosis as proposed by Balitzer [14] was 0 in 28 (43.0%) cases, 1 in 12 (18.5%) cases, and 2 in 25 (38.5%) cases.
Mean simplified AIH score [7] was 5.8 ± 1.3 (range 3–8) in the overall series, with 26 (40.0%) patients in the non-probable category (score ≤ 5), 20 (30.8%) in the probable category (score = 6), and 18 (27.7%) in the definite category (score ≥ 7). As expected, 22 out of 26 (84.6%) patients with low score were in the FUP group, while 15 out of 19 (78.9%) FPB received a score ≥ 6. One FBP patient (from another institute) did not receive a simplified AIH score, since serum data were not complete.
Table 2 shows the differences in histology between the two patient groups. As expected, most of the histopathological features of active inflammation were significantly higher in FBP than in FUP, including the grade of portal inflammation (p = 0.005, chi-square test), interface hepatitis (p = 0.001), and lobular inflammation (p = 0.001); the occurrence of Councilman bodies (p = 0.001); and the plasma cells count (p = 0.010, Mann–Whitney test). Also, emperipolesis was more represented in FBP (p = 0.002), while hepatocytic rosettes and “cobblestone” appearance were distributed equally in all cases. AIH score was 2 in 13 out of 20 FBP (65.0%) and in 12 out of 45 (26.7%) FUP (p = 0.005).
FBP showed worse portal fibrosis (p = 0.001, Mann–Whitney), with 17/20 (85.0%) cases with stage ≥ 2 according to Ishak versus 26 (57.8%) FUP cases with stage ≥ 2. Thus, FUP averagely showed a better control on disease progression, despite the time from the first diagnosis.
Histology of bile ducts in active AIH
Bile duct changes occurred in 70% of FBP and 33.3% of FUP (p = 0.023), and they were strongly related to the grade portal inflammation (p < 0.001, Spearman’s test) and portal fibrosis (p = 0.003). Hepatocytic K7 immunoreactivity together with focal copper deposits was observed in 6 (9.2%) cases. In line with what was previously reported [14], the bile duct changes observed in AIH seem to be directly related to the intensity of the inflammatory portal infiltrate rather than a direct bile duct aggression (Fig. 1).
A portal tract in a case of active autoimmune hepatitis, characterized by intense inflammatory infiltrate and bile duct alterations, without direct aggression. In the lower-left square, immunohistochemistry for Keratin 7 showing ductular reaction without hepatocytic biliary metaplasia. Magnification × 20
Portal lymphocytic apoptosis
Lymphocytic apoptotic bodies were evaluated in 3 portal hot spots for each case: the mean was 6.4 ± 5.7 (range 0–26). Since the apoptotic body count was higher in FBP (9.45 ± 6.95) than in FUP (5.00 ± 4.50, p = 0.002, t-test), it resulted in a correlation to the inflammation, indeed (Fig. 2). In particular, mean apoptosis number was correlated positively with the grade of portal inflammation (p < 0.001, Mann–Whitney test), the grade of interface hepatitis (p < 0.001), plasma cell number (p = 0.001), lobular inflammation (p < 0.001), and Councilman bodies (p = 0.001). As a result, mean apoptosis number correlated with AIH score as well (p = 0.003, ANOVA test), but also with emperipolesis (p = 0.003, Mann–Whitney test), bile duct alterations (p = 0.001), AST (p = 0.004), and ALT (p = 0.031) levels. Consequently, the 28 patients who received a low histological score according to Balitzer had a lower mean apoptotic count than the others (3.54 versus 8.01): nevertheless, 6 (21.4%) of these patients had a high mean apoptotic count (≥ 5).
To compare results of the apoptotic count, we chose other liver disease models: PBC as another model of autoimmune liver damage, as well as drugs and HCV as models of active inflammatory liver damage (Fig. 3). The mean apoptotic body counts in PBC, HCV, and DILI cases were 3.50 ± 1.67, 5.92 ± 3.44, and 3.18 ± 1.41, respectively. These values were comparable to apoptotic count in FUP group (p = 0.306, p = 0.874, and p = 0.219, respectively, t-test), but significantly lower than FBP group (p = 0.013, p = 0.004, and p = 0.010, respectively). Of note, the grade portal inflammation was comparable in the control groups as well as in AIH cases, with a mean Ishak’s grade of 1.50, 1.67, and 1.60 in PBC, HCV, and DILI cases, as well as 1.16 and 2.05 in FUP and FPB cases. As a result, the mean apoptotic count was significantly different between AIH cases and overall control cases (p = 0.025), despite the grade of inflammatory infiltrate (p = 0.254).
Can lymphocytic apoptosis be useful among the criteria of the simplified AIH score?
Interesting results came from the comparison between apoptotic count and simplified AIH score. In the overall AIH cases, the mean apoptotic count increased with the AIH score, with a mean of 3.98 ± 3.35, 7.70 ± 6.60, and 8.45 ± 6.56 in cases with an AIH score of 5, 6, and ≥ 7, respectively (p = 0.01 ANOVA test).
In the FBP cases, who did not receive any therapy, the mean apoptotic count was 6.50 ± 6.35, 9.23 ± 7.20, and 11.84 ± 7.49, respectively, with no statistical differences among the non-probable, probable, and definite categories (p = 0.481). Using the abovementioned cutoff value of ≥ 5 mean apoptotic bodies, arbitrarily chosen based on AIH activity and Balitzer score, we tried to simulate how this new histological feature can modify the simplified AIH score in our 19 FBP (1 patient did not have enough clinical data to assess the score).
Among the 4 FBP in the “non-probable category” (simplified AIH score ≤ 5), 2 (50%) had a mean apoptotic body count > 5: if we assign 1 point to the simplified AIH score, both cases would shift to the “probable” category.
The final number of FPB in the “non-probable” category would result in 2.
Among the 7 FBP in the “probable” category (simplified AIH score = 6), 5 (71%) cases had a mean apoptotic body count > 5 and would shift to the “definite” category.
The final number of FPB in the “probable” category would result in 4 (considering the 2 cases from the “non-probable” category).
All 8 (100%) FPB already in the “definite” category had a mean apoptotic body count > 5.
The final number of FPB in the “definite” category would results in 13.
In this simulation, assigning 1 point of score to FBP with > 5 mean apoptotic body count, the patients in the “definite” category after the first biopsy would have been 68% (instead of 42%).
Discussion
Histopathological analysis plays a key role in the diagnosis of AIH since the formulation of the first diagnostic scores [5]. However, albeit the single histopathological criteria for AIH are well-established, their reproducibility is questionable. Recently, confirming the importance of many criteria but questioning others, such as emperipolesis, some researchers revised the role of histopathology in AIH diagnosis [14]. Moreover, the authors confirmed the importance of excluding bile duct disease, utilizing also ancillary techniques, and proposed a score for the histopathological grading of AIH [14]. Our work confirms once more that plasma cell number and portal inflammation correlate with the AIH activity, together with other single histopathological features. These “classic” AIH criteria are strongly associated with each other as well. Also, emperipolesis was significantly more represented in the FBP and showed a correlation with an acute AIH presentation: emperipolesis is a sign of acute hepatitis, poorly specific of AIH, and difficult to define without ancillary techniques [14]. Other features such as hepatocytic rosettes and “cobblestone” appearance [15] were represented equally in both the FBP and FUP groups, demonstrating less correlation with disease activity: they might be considered the most sensitive of AIH (despite inflammatory activity), albeit they are seen as less specific [14].
In our series, a peculiar characteristic of the FBP group (sent to the TRC because of clinical data suggesting autoimmune disease) was a significantly higher fibrosis stage than FUP (with a previous diagnosis of AIH in other hospitals), meaning a more advanced disease progression at the time of the first diagnosis. This finding could derive from a delayed or underestimated AIH diagnosis in patients’ history, before coming to the TRC for an acute flare. At any chance, it confirms our previous experience on a multicenter Italian study: we studied AIH clinical acute onset, subdividing the “genuine” acute onset based on a low degree of fibrosis at the histological level and identifying patients with Ishak’s stage ≥ 3 as “acute on chronic” [21]. Here, we restate that the degree of fibrosis at diagnosis could represent the gold standard to define an actual acute AIH onset versus a reactivation of a chronic picture (i.e., acute-on-chronic AIH).
Bile duct changes were defined as alterations in shape and size of bile ducts, with focal nuclear irregularity [20]. Following this definition, biliary injury can be focally present, and it is reported in up to 12% of AIH by the literature [20, 22]. Balitzer et al. suggested the use of K7 and copper deposits to exclude chronic biliary damage [14] since biliary damage has been considered a negative factor in AIH diagnostic score [7]. However, some authors postulated that once the coexistence of chronic biliary diseases is excluded, AIH diagnosis should not be excluded based on biliary changes [22, 23]. Applying the most recent indications, in our series, we found morphological signs of bile duct changes in 40 (61.5%) patients, and in particular in 70% of FBP and 33% of FUP. This incidence is in line with most recent observations [24], together with the finding that bile duct alterations were strongly related to portal inflammation (p < 0.001), rather than with a direct bile duct aggression. A possible explanation is that a heavy chronic inflammatory infiltrate causes vascular congestion due to portal tract compression. The biliary regressive changes could be a consequence of transient hypoperfusion.
In addition to the validation of the “classic” AIH criteria, another important aim of the present study was to apply the count of apoptotic bodies in the portal tracts as a novel feature for AIH diagnosis. Our results showed that lymphocyte apoptosis in portal tracts is a constant event in untreated AIH. Here in line with recent observations, in this report remains indicated that apoptosis of Treg circulating lymphocytes has specifically increased in active AIH [25]. In a mouse model, it has been demonstrated that Tregs are highly sensitive to apoptosis, associated with a low expression of the anti-apoptotic molecule and the development of autoimmunity [26]. In our data, the apoptotic count in the portal tracts is directly correlated with the main variables of inflammatory activity, as well as with the AIH grading score proposed by Balitzer et al. [14], confirming that the increase of apoptotic activity is proportional to the grade of inflammation. We found a high apoptotic count, defined as ≥ 5, in more than 21% of the cases with a low histological score for AIH diagnosis. Using the same cutoff, we simulated the simplified AIH score for our FBP, assigning 1 point in the presence of > 5 apoptotic body count: as a consequence, the percentage of FBP who would be put in the “definite” category raises from 42 to 68%. The aforementioned encourages our feeling that apoptosis might be considered a histological criterion in AIH diagnosis. Due to the lack of a gold standard to be compared with, the choice of the cutoff was arbitrary and it was mainly based on the comparison with the controls. Further analyses are required to establish an eventual diagnostic cutoff to be applied in the AIH diagnostic score.
Studies on other autoimmune diseases and hyperimmune conditions demonstrated a dysregulation in apoptotic mechanisms [18, 27, 28]. Many mechanisms play a role in apoptosis dysregulation in LES, including the survival of self-reactive B lymphocytes promoted by the lack of apoptotic body phagocytosis [27]. Another model described an increased self-reactivity of the B lymphocytes by an impairment of T-helper lymphocytes control and survival mechanisms [28]. To compare our findings with other mechanisms of liver disease, we counted lymphocytic apoptosis in the portal tracts of 25 HCV-related acute hepatitis, 10 PBC, and 10 DILI: the mean apoptotic count in FUP was comparable to control cases, albeit PBC cases showed much lower apoptotic bodies (Fig. 3). FBP cases showed a significantly higher apoptotic count than control cases, independently of the amount of portal inflammatory infiltrate: this finding could suggest that in different active liver diseases with a comparable amount of inflammation, the lymphocyte turnover might be dissimilar. Further studies focusing on the T-cell gene expression are required.
The count of apoptotic bodies on biopsy is used already in other clinical settings, such as in acute cellular rejection of bowel transplantation, where an increase in crypt epithelial cell apoptosis is a significant histological criterion for the diagnosis [28]. So far, the relationship between inflammatory cell apoptosis and AIH has not been investigated. Our results confirm that the increased apoptotic activity in portal spaces in AIH represents a sign of dysregulated lymphocyte turnover [22], especially in acute AIH. Moreover, apoptotic count in FBP cases remains high in all the diagnostic categories as calculated with the simplified AIH score, and especially between the “probable” and “definite” categories, suggesting that the apoptotic count could be proposed as diagnostic criteria for acute AIH, especially in first biopsies from not treated patients, also without any other typical histological features.
In conclusion, apoptotic count seems to represent a new morphological feature in the diagnosis of acute AIH as in the flare of acute-on-chronic AIH. The connection between inflammatory cell apoptosis and AIH needs further investigation. As previously mentioned, the results show how the increased apoptotic activity in portal spaces in AIH expresses a sign of dysregulated lymphocyte turnover [22], primarily in acute AIH. The apoptotic count can be proposed as a diagnostic criterion for AIH in the acute phase, especially in first biopsies without other classic features, and could be included among the future histological variables of the AIH score, providing a definite cutoff value.
References
Lv T, Li M, Zeng N, Zhang J, Li S, Chen S, Zhang C, Shan S, Duan W, Wang Q, Wu S, You H, Ou X, Ma H, Zhang D, Kong Y, Jia J (2019) Systematic review and meta-analysis on the incidence and prevalence of autoimmune hepatitis in Asian, European, and American population. J Gastroenterol Hepatol 34:1676–1684. https://doi.org/10.1111/jgh.14746
Werner M, Prytz H, Ohlsson B, Almer S, Björnsson E, Bergquist A, Wallerstedt S, Sandberg-Gertzén H, Hultcrantz R, Sangfelt P, Weiland O, Danielsson A (2008) Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol 43:1232–1240. https://doi.org/10.1080/00365520802130183
van Gerven NM, Verwer BJ, Witte BI, van Erpecum KJ, van Buuren HR, Maijers I, Visscher AP, Verschuren EC, van Hoek B, Coenraad MJ, Beuers UH, de Man RA, Drenth JP, den Ouden JW, Verdonk RC, Koek GH, Brouwer JT, Guichelaar MM, Vrolijk JM, Mulder CJ, van Nieuwkerk CM, Bouma G (2014) Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand J Gastroenterol 49:1245–1254. https://doi.org/10.3109/00365521.2014.946083
Strettell MD, Donaldson PT, Thomson LJ, Santrach PJ, Moore SB, Czaja AJ, Williams R (1997) Allelic basis for HLA-encoded susceptibility to type 1 autoimmune hepatitis. Gastroenterology 112:2028–2035. https://doi.org/10.1053/gast.1997.v112.pm9178696
Johnson PJ, McFarlane IG (1993) Meeting report: International Autoimmune Hepatitis Group. Hepatology 18:998–1005. https://doi.org/10.1002/hep.1840180435
Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston AL, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RN, Maddrey WC, Manns MP, McFarlane IG, Meyer zumBüschenfelde KH, Zeniya M et al (1999) International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 31:929–938. https://doi.org/10.1016/s0168-8278(99)80297-9
Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW (2008) International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 48:169–176. https://doi.org/10.1002/hep.22322
McFarlane IG (2002) Autoimmune hepatitis: diagnostic criteria, subclassifications, and clinical features. Clin Liver Dis 6:605–621. https://doi.org/10.1016/s1089-3261(02)00019-3
Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ (2005) Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology 42:53–62. https://doi.org/10.1002/hep.20732
Vergani D, Alvarez F, Bianchi FB, Cançado EL, Mackay IR, Manns MP, Nishioka M, Penner E (2004) Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol 41:677–683. https://doi.org/10.1016/j.jhep.2004.08.002
Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM (2010) Diagnosis and management of autoimmune hepatitis. Hepatology 51:2193–2213. https://doi.org/10.1002/hep.23584
European Association for the Study of the Liver (2015) EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol 63:971–1004. https://doi.org/10.1016/j.jhep.2015.06.030
Czaja AJ, Carpenter HA (1993) Sensitivity, specificity, and predictability of biopsy interpretations in chronic hepatitis. Gastroenterology 105:1824–1832. https://doi.org/10.1016/0016-5085(93)91081-r
Balitzer D, Shafizadeh N, Peters MG, Ferrell LD, Alshak N, Kakar S (2017) Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Mod Pathol 30:773–783. https://doi.org/10.1038/modpathol.2016.267
Nguyen Canh H, Harada K, Ouchi H, Sato Y, Tsuneyama K, Kage M, Nakano M, Yoshizawa K, Takahashi A, Abe M, Kang JH, Koike K, Inui A, Fujisawa T, Takaki A, Arinaga-Hino T, Torimura T, Suzuki Y, Fujiwara K, Zeniya M, Ohira H, Tanaka A, Takikawa H (2017) Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol 70:961–969. https://doi.org/10.1136/jclinpath-2016-204271
Tsutsui A, Harada K, Tsuneyama K, Senoh T, Nagano T, Takaguchi K, Ando M, Nakamura S, Mizobuchi K, Kudo M (2017) Clinicopathological study of autoimmune hepatitis cases that were difficult to differentiate from drug-induced liver injury. Dig Dis 35:506–514. https://doi.org/10.1159/000480139
Svensson BO (1975) Serum factors causing impaired macrophage function in systemic lupus erythematosus. Scand J Immunol 4:145–150. https://doi.org/10.1111/j.1365-3083.1975.tb02611.x
Ruiz P, Bagni A, Brown R, Cortina G, Harpaz N, Magid MS, Reyes J (2004) Histological criteria for the identification of acute cellular rejection in human small bowel allografts: results of the pathology workshop at the VIII International Small Bowel Transplant Symposium. Transplant Proc 36:335–337. https://doi.org/10.1016/j.transproceed.2004.01.079
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN et al (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22:696–699. https://doi.org/10.1016/0168-8278(95)80226-6
Burt A, Ferrell L, Hubscher S (2017) MacSween’s pathology of the liver, 7th edn. Elsevier
Muratori P, Carbone M, Stangos G, Perini L, Lalanne C, Ronca V, Cazzagon N, Bianchi G, Lenzi M, Floreani A, Invernizzi P, Muratori L (2018) Clinical and prognostic implications of acute onset of autoimmune hepatitis: an Italian multicentre study. Dig Liver Dis 50:698–702. https://doi.org/10.1016/j.dld.2018.02.015
Czaja AJ, Carpenter HA (2001) Autoimmune hepatitis with incidental histologic features of bile duct injury. Hepatology 34:659–665. https://doi.org/10.1053/jhep.2001.27562
Czaja AJ, Muratori P, Muratori L, Carpenter HA, Bianchi FB (2004) Diagnostic and therapeutic implications of bile duct injury in autoimmune hepatitis. Liver Int 24:322–329. https://doi.org/10.1111/j.1478-3231.2004.0924.x
Shen Y, Lu C, Men R, Liu J, Ye T, Yang L (2018) Clinical and pathological characteristics of autoimmune hepatitis with acute presentation. Can J Gastroenterol Hepatol 2018:3513206. https://doi.org/10.1155/2018/3513206
John K, Hardtke-Wolenski M, Jaeckel E, Manns MP, Schulze-Osthoff K, Bantel H (2017) Increased apoptosis of regulatory T cells in patients with active autoimmune hepatitis. Cell Death Dis 8:3219. https://doi.org/10.1038/s41419-017-0010-y
Yüksel M, Laukens D, Heindryckx F, Van Vlierberghe H, Geerts A, Wong FS, Wen L, Colle I (2014) Hepatitis mouse models: from acute-to-chronic autoimmune hepatitis. Int J Exp Pathol 95:309–320. https://doi.org/10.1111/iep.12090
Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M (2002) Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum 46:191–201. https://doi.org/10.1002/1529-0131(200201)46:1%3c191::AID-ART10027%3e3.0.CO;2-K
Riella LV, Paterson AM, Sharpe AH, Chandraker A (2012) Role of the PD-1 pathway in the immune response. Am J Transplant 12:2575–2587. https://doi.org/10.1111/j.1600-6143.2012.04224.x
Author information
Authors and Affiliations
Contributions
TF: study concept and design, acquisition of data, article drafting, final approval of the version to be published.
FV: study concept and design, analysis of data, article drafting, final approval of the version to be published.
PM: acquisition of data, critical revision of the article for important intellectual contents, final approval of the version to be published.
LM: analysis of data, critical revision of the article for important intellectual contents, final approval of the version to be published.
MG: analysis of data, article drafting, final approval of the version to be published.
ML: acquisition of data, critical revision of the article for important intellectual contents, final approval of the version to be published.
AD: study concept and design, article drafting, final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics approval
This is a monocentric retrospective study on archival tissue, taken in course of routine diagnostic procedures. Patients signed inform consent and they were treated anonymously according to the Ethical Guidelines of the 1975 Declaration of Helsinki (6th revision 2008).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Franceschini, T., Vasuri, F., Muratori, P. et al. A practical histological approach to the diagnosis of autoimmune hepatitis: experience of an Italian tertiary referral center. Virchows Arch 479, 937–945 (2021). https://doi.org/10.1007/s00428-021-03122-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-021-03122-5