Abstract
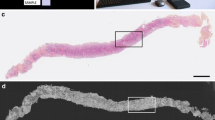
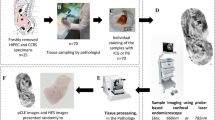
Ex vivo fluorescence confocal microscopy (FCM) is an optical technology that provides fast H&E-like images of freshly excised tissues, and it has been mainly used for “real-time” pathological examination of dermatological malignancies. It has also shown to be a promising tool for fast pathological examination of prostatic tissues. We aim to create an atlas for FCM images of prostatic and periprostatic tissues to facilitate the interpretation of these images. Furthermore, we aimed to evaluate the learning curve of images interpretation of this new technology. Eighty fresh and unprepared biopsies obtained from radical prostatectomy specimens were evaluated using the FCM VivaScope® 2500 M-G4 (Mavig GmbH, Munich, Germany; Caliber I.D.; Rochester NY, USA) by two pathologists. Images of FCM with the corresponding H&E are illustrated to create the atlas. Furthermore, the two pathologists were asked to re-evaluate the 80 specimens after 90 days interval in order to assess the learning curve of images’ interpretation of FCM. FCM was able to differentiate between different types of prostatic and periprostatic tissues including benign prostatic glands, benign prostatic hyperplasia, high-grade intraepithelial neoplasm, and prostatic adenocarcinoma. As regards the learning curve, FCM demonstrated a short learning curve. We created an atlas that can serve as the base for urologists and pathologists for learning and interpreting FCM images of prostatic and periprostatic tissues. Furthermore, FCM images is easily interpretable; however, further studies are required to explore the potential applications of this new technology in prostate cancer diagnosis and management.




Similar content being viewed by others
References
Alshieban S, Al-Surimi K (2015) Reducing turnaround time of surgical pathology reports in pathology and laboratory medicine departments. BMJ Qual Imp Rep 4:u209223–w203773. https://doi.org/10.1136/bmjquality.u209223.w3773
Avellini C, Baccarani U, Orsaria M, Adani GL, Bresadola V, Lorenzin D, Bresadola F, Beltrami CA (2009) Evaluation of prostate cancer staging in organ donors: intraoperative histology on periglandular soft tissues-a proposal. Transplant Proc 41:1099–1103. https://doi.org/10.1016/j.transproceed.2009.03.089
Bertoni L, Azzoni P, Reggiani C, Pisciotta A, Carnevale G, Chester J, Kaleci S, Reggiani Bonetti L, Cesinaro AM, Longo C, Pellacani G (2018) Ex vivo fluorescence confocal microscopy for intraoperative, real-time diagnosis of cutaneous inflammatory diseases: a preliminary study. Exp Dermatol 27:1152–1159. https://doi.org/10.1111/exd.13754
Bertoni L, Pisciotta A, Azzoni P, Bertani G, Reggiani Bonetti L, Puliatti S, Farnetani F, Carnevale G, Pellacani G (2018) Use of ex vivo fluorescence confocal microscopy for detection of tissue specific markers. Biomed J Sci & Tech Res 10. https://doi.org/10.26717/BJSTR.2018.10.002003
Edgar B (2004) Applied nonparametric statistical methods. P. Sprent and N. C. Smeeton, Chapman & Hall/CRC, London, England, 2001. No. of pages: ix+461. Price:£29.99. ISBN: 1-584-88145-3 Statistics in medicine 23:1988-1989. https://doi.org/10.1002/sim.1755
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA (2016) The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 40:244–252. https://doi.org/10.1097/pas.0000000000000530
Fleiss JL (1981) The measurement of interrater agreement.Statistical Methods for Rates and Proportions, 2nd Edition.Edn. Wiley, NewYork, pp 212–236
Gareau DS, Li Y, Huang B, Eastman Z, Nehal KS, Rajadhyaksha M (2008) Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt 13:054001. https://doi.org/10.1117/1.2981828
Hartmann D, Krammer S, Bachmann MR, Mathemeier L, Ruzicka T, Bagci IS, von Braunmuhl T (2018) Ex vivo confocal microscopy features of cutaneous squamous cell carcinoma. J Biophotonics 11:e201700318. https://doi.org/10.1002/jbio.201700318
Hartmann D, Krammer S, Ruini C, Ruzicka T, von Braunmuhl T (2016) Correlation of histological and ex-vivo confocal tumor thickness in malignant melanoma. Lasers Med Sci 31:921–927. https://doi.org/10.1007/s10103-016-1936-5
Hartmann D, Krammer S, Vural S, Bachmann MR, Ruini C, Sardy M, Ruzicka T, Berking C, von Braunmuhl T (2018) Immunofluorescence and confocal microscopy for ex-vivo diagnosis of melanocytic and non-melanocytic skin tumors: a pilot study. J Biophotonics:11. https://doi.org/10.1002/jbio.201700211
Hartmann D, Ruini C, Mathemeier L, Dietrich A, Ruzicka T, von Braunmuhl T (2016) Identification of ex-vivo confocal scanning microscopic features and their histological correlates in human skin. J Biophotonics 9:376–387. https://doi.org/10.1002/jbio.201500124
Hastie T, Tibshirani R, Friedman J (2009) The elements of statistical learning data mining, inference, and prediction. Springer Verlag, Secaucus
Jain M, Pulijal SV, Rajadhyaksha M, Halpern AC, Gonzalez S (2018) Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Derm 154:962–965. https://doi.org/10.1001/jamadermatol.2018.1668
Kuiper T, Kiesslich R, Ponsioen C, Fockens P, Dekker E (2012) The learning curve, accuracy, and interobserver agreement of endoscope-based confocal laser endomicroscopy for the differentiation of colorectal lesions. Gastrointest Endosc 75:1211–1217. https://doi.org/10.1016/j.gie.2012.01.040
Liu J, Li M, Li Z, Zuo XL, Li CQ, Dong YY, Zhou CJ, Li YQ (2014) Learning curve and interobserver agreement of confocal laser endomicroscopy for detecting precancerous or early-stage esophageal squamous cancer. PLoS One 9:e99089. https://doi.org/10.1371/journal.pone.0099089
Longo C, Borsari S, Pampena R, Benati E, Bombonato C, Raucci M, Mirra M, Di Stefani A, Peris K, Pellacani G (2018) Basal cell carcinoma: the utility of in vivo and ex vivo confocal microscopy. J Eur Acad Dermatol Venereol 32:2090–2096. https://doi.org/10.1111/jdv.14984
Lopez A, Zlatev DV, Mach KE, Bui D, Liu JJ, Rouse RV, Harris T, Leppert JT, Liao JC (2016) Intraoperative optical biopsy during robotic assisted radical prostatectomy using confocal endomicroscopy. J Urol 195:1110–1117. https://doi.org/10.1016/j.juro.2015.10.182
Magi-Galluzzi C (2018) Prostate cancer: diagnostic criteria and role of immunohistochemistry Modern pathology : an official journal of the United States and Canadian. Acad Pathol, Inc 31:S12–S21. https://doi.org/10.1038/modpathol.2017.139
MAVIG (2018) Datasheet VivaScope® 2500M-G4 https://www.vivascope.de/wp-content/uploads/2019/06/DS_VS-2500M-G4_287_0219-ohne-Mohs.pdf. Accessed 4 January 2020
Morgan MS, Lay AH, Wang X, Kapur P, Ozayar A, Sayah M, Zeng L, Liu H, Roehrborn CG, Cadeddu JA (2016) Light reflectance spectroscopy to detect positive surgical margins on prostate cancer specimens. J Urol 195:479–483. https://doi.org/10.1016/j.juro.2015.05.115
Obek C, Saglican Y, Ince U, Argun OB, Tuna MB, Doganca T, Tufek I, Keskin S, Kural AR (2018) Intra-surgical total and re-constructible pathological prostate examination for safer margins and nerve preservation (Istanbul preserve). Ann Diagn Pathol 33:35–39. https://doi.org/10.1016/j.anndiagpath.2017.11.010
Panarello D, Comperat E, Seyde O, Colau A, Terrone C, Guillonneau B (2019) Atlas of ex vivo prostate tissue and cancer images using confocal laser endomicroscopy: a project for intraoperative positive surgical margin detection during radical prostatectomy. Eur Urol Focus. https://doi.org/10.1016/j.euf.2019.01.004
Puliatti S, Bertoni L, Pirola GM, Azzoni P, Bevilacqua L, Eissa A, Elsherbiny A, Sighinolfi MC, Chester J, Rocco B, Micali S, Bagni I, Reggiani Bonetti L, Maiorana A, Malvehy J, Longo C, Montironi R, Bianchi G, Pellacani G (2019) Ex-vivo fluorescence confocal microscopy: the first application for real-time pathologic examination of prostatic tissue. BJU Int. https://doi.org/10.1111/bju.14754
Ragazzi M, Longo C, Piana S (2016) Ex vivo (fluorescence) confocal microscopy in surgical pathology: state of the art. Adv Anat Pathol 23:159–169. https://doi.org/10.1097/pap.0000000000000114
Ragazzi M, Piana S, Longo C, Castagnetti F, Foroni M, Ferrari G, Gardini G, Pellacani G (2014) Fluorescence confocal microscopy for pathologists Modern pathology : an official journal of the United States and Canadian. Acad Pathol, Inc 27:460–471. https://doi.org/10.1038/modpathol.2013.158
Samaratunga H, Montironi R, True L, Epstein JI, Griffiths DF, Humphrey PA, van der Kwast T, Wheeler TM, Srigley JR, Delahunt B, Egevad L (2011) International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 1: specimen handling Modern pathology : an official journal of the United States and Canadian. Acad Pathol, Inc 24:6–15. https://doi.org/10.1038/modpathol.2010.178
Schlomm T, Tennstedt P, Huxhold C, Steuber T, Salomon G, Michl U, Heinzer H, Hansen J, Budaus L, Steurer S, Wittmer C, Minner S, Haese A, Sauter G, Graefen M, Huland H (2012) Neurovascular structure-adjacent frozen-section examination (NeuroSAFE) increases nerve-sparing frequency and reduces positive surgical margins in open and robot-assisted laparoscopic radical prostatectomy: experience after 11,069 consecutive patients. Eur Urol 62:333–340. https://doi.org/10.1016/j.eururo.2012.04.057
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019 CA Cancer J Clin 69:7–34. doi: https://doi.org/10.3322/caac.21551
Tewari AK, Shevchuk MM, Sterling J, Grover S, Herman M, Yadav R, Mudalair K, Srivastava A, Rubin MA, Zipfel WR, Maxfield FR, Xu C, Webb WW, Mukherjee S (2011) Multiphoton microscopy for structure identification in human prostate and periprostatic tissue: implications in prostate cancer surgery. BJU Int 108:1421–1429. https://doi.org/10.1111/j.1464-410X.2011.10169.x
Wang M, Kimbrell HZ, Sholl AB, Tulman DB, Elfer KN, Schlichenmeyer TC, Lee BR, Lacey M, Brown JQ (2015) High-resolution rapid diagnostic imaging of whole prostate biopsies using video-rate fluorescence structured illumination microscopy. Cancer Res 75:4032–4041. https://doi.org/10.1158/0008-5472.can-14-3806
Wang M, Tulman DB, Sholl AB, Kimbrell HZ, Mandava SH, Elfer KN, Luethy S, Maddox MM, Lai W, Lee BR, Brown JQ (2016) Gigapixel surface imaging of radical prostatectomy specimens for comprehensive detection of cancer-positive surgical margins using structured illumination microscopy. Sci Rep 6:27419. https://doi.org/10.1038/srep27419
Author information
Authors and Affiliations
Contributions
Bertoni L., Puliatti S., Eissa A., Sighinolfi MC, Bianchi G, Pellacani G, Rocco B, Montironi R: Conception and Design. Bertoni L, Puliatti S, Regianni Bonetti L, Maiorana A, Azzoni P, Bevilacqua L, Spandri V, Montironi R: Acquisition of Data. Kaleci S: Data Analysis. Bertoni L, Puliatti S, Regianni Bonetti L, Maiorana A, Eissa A, Azzoni P, Sighinolfi MC, Micali S, Rocco B, Montironi R: Interpretation of Data. Bertoni L, Puliatti S, Regianni Bonetti L, Eissa A, Zoeir A, Sighinolfi MC: Drafting and writing. Micali S, Bianchi G, Pellacani G, Rocco B, Montironi R: Revision
Corresponding author
Ethics declarations
This study was approved by the Ethical Committee in the university of Modena & Reggio Emilia (protocol number 0018091/18) and written informed consent was obtained from all patients.
Conflict of interest
Eissa A has a temporary contract of consultation with MAVIG GmbH.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
Misdiagnosed prostatic adenocarcinoma (A-F). In histological image of focus of Prostatic Acinar Adenocarcinoma Grade Group 1, nuclei were enlarged and nucleoli were evident (A), differently from FCM correlate in which glands exhibited mild atypia and pluristratified crowded nuclei resembling HG-PIN and the atypia was not correctly evidenced (B). Adenocarcinoma was masqueraded by technical artifact: in histological image, the atypical glands were recognizable (C), but not distinguishable in FCM images (D). Cord and single cell of undifferentiated Grade Group 4 adenocarcinoma were visible in histological image (E) differently from FCM corresponding imagine that showed little and pale nuclei misdiagnosed as inflammatory infiltrate (F). (Scale bar = 50 μm) (PDF 13936 kb)
Rights and permissions
About this article
Cite this article
Bertoni, L., Puliatti, S., Reggiani Bonetti, L. et al. Ex vivo fluorescence confocal microscopy: prostatic and periprostatic tissues atlas and evaluation of the learning curve. Virchows Arch 476, 511–520 (2020). https://doi.org/10.1007/s00428-019-02738-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02738-y