Abstract
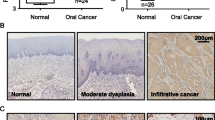
Phosphatidylinositol-3-kinases are kinases that lead to AKT phosphorylation and thus mTOR and GSK3β activation. These proteins are linked to tumorigenesis, but their roles in driving cervical lymph node (CLN) metastasis of oral squamous cell carcinoma (OSCC) cells are unknown. This study aimed to investigate the role of AKT, mTOR, and GSK3β proteins in the occurrence of CLN metastasis in OSCC patients. Ninety and 18 paraffin-embedded OSCC and oral mucosa samples were included, respectively. We divided our OSCC patients into non-metastasizing (PNM) and metastasizing (PM) groups, and the expression of total AKT, pAKT1Thr308, pAKTSer473, GSK3β, pGSK3βSer9, and pmTORSer2448 was analyzed by immunohistochemistry. The mean expression of GSK3β, pGSK3βSer9, total AKT, and pmTOR2448 was always higher in the OSCC tissues than that in the controls. A positive correlation was also found among these proteins. Total AKT, pmTORSer2448, and pGSK3βSer9 expression was significantly higher in the PNM and PM groups than that in the control group. However, only GSK3β expression was significantly higher in the PM group compared with the PNM group. High expression levels of GSK3β and pGSK3βSer9 were significantly associated with CLN metastasis, but only GSK3β remained an independent predictor of CLN metastasis. pGSK3βSer9 and CLN metastasis were associated with a poor prognosis, but only the latter remained an independent prognostic parameter. Kaplan-Meier survival curves showed that pGSK3βSer9 and CLN metastasis were significantly related to reduced survival rates. These results suggest that AKT and mTOR proteins are involved in OSCC biology and that GSK3β itself may drive CLN metastatic spread of OSCC cells.






Similar content being viewed by others
References
Simard EP, Torre LA, Jemal A (2014) International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol 50(5):387–403. https://doi.org/10.1016/j.oraloncology.2014.01.016
Genden EM, Ferlito A, Silver CE, Takes RP, Suarez C, Owen RP, Haigentz M Jr, Stoeckli SJ, Shaha AR, Rapidis AD, Rodrigo JP, Rinaldo A (2010) Contemporary management of cancer of the oral cavity. Eur Arch Otorhinolaryngol 267(7):1001–1017. https://doi.org/10.1007/s00405-010-1206-2
Giudice FS, Squarize CH (2013) The determinants of head and neck cancer: unmasking the PI3K pathway mutations. J Carcinog Mutagen Suppl 5. doi:https://doi.org/10.4172/2157-2518.S5-003
Biazevic MG, Castellanos RA, Antunes JL, Michel-Crosato E (2006) Trends in oral cancer mortality in the city of Sao Paulo, Brazil, 1980-2002. Cad Saude Publica 22(10):2105–2114
Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB (1994) Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer 73(1):187–190
Cooper JS, Porter K, Mallin K, Hoffman HT, Weber RS, Ang KK, Gay EG, Langer CJ (2009) National Cancer Database report on cancer of the head and neck: 10-year update. Head Neck 31(6):748–758. https://doi.org/10.1002/hed.21022
Hedberg ML, Goh G, Chiosea SI, Bauman JE, Freilino ML, Zeng Y, Wang L, Diergaarde BB, Gooding WE, Lui VW, Herbst RS, Lifton RP, Grandis JR (2016) Genetic landscape of metastatic and recurrent head and neck squamous cell carcinoma. J Clin Invest 126(4):1606. https://doi.org/10.1172/JCI86862
Wong KK, Engelman JA, Cantley LC (2010) Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev 20(1):87–90. https://doi.org/10.1016/j.gde.2009.11.002
Cully M, You H, Levine AJ, Mak TW (2006) Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 6(3):184–192. https://doi.org/10.1038/nrc1819
Engelman JA (2009) Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer 9(8):550–562. https://doi.org/10.1038/nrc2664
Kozaki K, Imoto I, Pimkhaokham A, Hasegawa S, Tsuda H, Omura K, Inazawa J (2006) PIK3CA mutation is an oncogenic aberration at advanced stages of oral squamous cell carcinoma. Cancer Sci 97(12):1351–1358. https://doi.org/10.1111/j.1349-7006.2006.00343.x
Qiu W, Schonleben F, Li X, Ho DJ, Close LG, Manolidis S, Bennett BP, Su GH (2006) PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res 12(5):1441–1446. https://doi.org/10.1158/1078-0432.CCR-05-2173
Fenic I, Steger K, Gruber C, Arens C, Woenckhaus J (2007) Analysis of PIK3CA and Akt/protein kinase B in head and neck squamous cell carcinoma. Oncol Rep 18(1):253–259
Lim J, Kim JH, Paeng JY, Kim MJ, Hong SD, Lee JI, Hong SP (2005) Prognostic value of activated Akt expression in oral squamous cell carcinoma. J Clin Pathol 58(11):1199–1205. https://doi.org/10.1136/jcp.2004.024786
Massarelli E, Liu DD, Lee JJ, El-Naggar AK, Lo Muzio L, Staibano S, De Placido S, Myers JN, Papadimitrakopoulou VA (2005) Akt activation correlates with adverse outcome in tongue cancer. Cancer 104(11):2430–2436. https://doi.org/10.1002/cncr.21476
Iamaroon A, Krisanaprakornkit S (2009) Overexpression and activation of Akt2 protein in oral squamous cell carcinoma. Oral Oncol 45(10):e175–e179. https://doi.org/10.1016/j.oraloncology.2009.06.003
Zheng S, Yang C, Lu M, Liu Q, Liu T, Dai F, Gao X, Ilyar S, Lu X (2016) PIK3CA promotes proliferation and motility but is unassociated with lymph node metastasis or prognosis in esophageal squamous cell carcinoma. Hum Pathol 53:121–129. https://doi.org/10.1016/j.humpath.2015.11.013
Du L, Shen J, Weems A, Lu SL (2012) Role of phosphatidylinositol-3-kinase pathway in head and neck squamous cell carcinoma. J Oncol 2012:450179–450112. https://doi.org/10.1155/2012/450179
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2(7):489–501. https://doi.org/10.1038/nrc839
Osaki M, Oshimura M, Ito H (2004) PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis 9(6):667–676. https://doi.org/10.1023/B:APPT.0000045801.15585.dd
Moral M, Paramio JM (2008) Akt pathway as a target for therapeutic intervention in HNSCC. Histol Histopathol 23(10):1269–1278
Vander Broek R, Mohan S, Eytan DF, Chen Z, Van Waes C (2015) The PI3K/Akt/mTOR axis in head and neck cancer: functions, aberrations, cross-talk, and therapies. Oral Dis 21(7):815–825. https://doi.org/10.1111/odi.12206
Mendonca DF, Chammas R, Liu FT, Nonogaki S, Cardoso SV, Loyola AM, de Faria PR (2012) The inactive form of glycogen synthase kinase-3beta is associated with the development of carcinomas in galectin-3 wild-type mice, but not in galectin-3-deficient mice. Int J Clin Exp Pathol 5(6):547–554
Mishra R (2010) Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer 9:144. https://doi.org/10.1186/1476-4598-9-144
Prasad CP, Rath G, Mathur S, Bhatnagar D, Parshad R, Ralhan R (2009) Expression analysis of E-cadherin, slug and GSK3beta in invasive ductal carcinoma of breast. BMC Cancer 9:325. https://doi.org/10.1186/1471-2407-9-325
Gao S, Li S, Duan X, Gu Z, Ma Z, Yuan X, Feng X, Wang H (2017) Inhibition of glycogen synthase kinase 3 beta (GSK3beta) suppresses the progression of esophageal squamous cell carcinoma by modifying STAT3 activity. Mol Carcinog 56(10):2301–2316. https://doi.org/10.1002/mc.22685
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127(3):469–480. https://doi.org/10.1016/j.cell.2006.10.018
Farago M, Dominguez I, Landesman-Bollag E, Xu X, Rosner A, Cardiff RD, Seldin DC (2005) Kinase-inactive glycogen synthase kinase 3beta promotes Wnt signaling and mammary tumorigenesis. Cancer Res 65(13):5792–5801. https://doi.org/10.1158/0008-5472.CAN-05-1021
Cardesa A, Gale N, Nadal A, Zidar N (2005) Tumors of the hypopharynx, larynx and trachea. In: Barnes L, Eveson JW, Reichart P, Sidransky D (eds) World Health Organization classification of tumors. Pathology and genetics of head and neck tumors. IARC, Lyon, pp 118–121
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4(7):844–847
Detre S, Saclani Jotti G, Dowsett M (1995) A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 48(9):876–878
Mishra R, Nagini S, Rana A (2015) Expression and inactivation of glycogen synthase kinase 3 alpha/beta and their association with the expression of cyclin D1 and p53 in oral squamous cell carcinoma progression. Mol Cancer 14:20. https://doi.org/10.1186/s12943-015-0300-x
Wang J, Wang X, Gong W, Mi B, Liu S, Jiang B (2009) Increased expression of beta-catenin, phosphorylated glycogen synthase kinase 3beta, cyclin D1, and c-myc in laterally spreading colorectal tumors. J Histochem Cytochem 57(4):363–371. https://doi.org/10.1369/jhc.2008.953091
Cortesina G, Martone T (2006) Molecular metastases markers in head and neck squamous cell carcinoma: review of the literature. Acta Otorhinolaryngol Ital 26(6):317–325
McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, Montalto G, D'Assoro AB, Libra M, Nicoletti F, Maestro R, Basecke J, Rakus D, Gizak A, Demidenko ZN, Cocco L, Martelli AM, Cervello M (2014) GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 5(10):2881–2911. https://doi.org/10.18632/oncotarget.2037
Zheng HC, Xu XY, Xia P, Yu M, Takahashi H, Takano Y (2010) Involvement of inactive GSK3beta overexpression in tumorigenesis and progression of gastric carcinomas. Hum Pathol 41(9):1255–1264. https://doi.org/10.1016/j.humpath.2010.02.003
Doble BW, Woodgett JR (2003) GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116(Pt 7):1175–1186
Baltzis D, Pluquet O, Papadakis AI, Kazemi S, Qu LK, Koromilas AE (2007) The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem 282(43):31675–31687. https://doi.org/10.1074/jbc.M704491200
Acosta-Jaquez HA, Keller JA, Foster KG, Ekim B, Soliman GA, Feener EP, Ballif BA, Fingar DC (2009) Site-specific mTOR phosphorylation promotes mTORC1-mediated signaling and cell growth. Mol Cell Biol 29(15):4308–4324. https://doi.org/10.1128/MCB.01665-08
Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24(50):7455–7464. https://doi.org/10.1038/sj.onc.1209085
Wu HT, Ko SY, Fong JH, Chang KW, Liu TY, Kao SY (2009) Expression of phosphorylated Akt in oral carcinogenesis and its induction by nicotine and alkaline stimulation. J Oral Pathol Med 38(2):206–213. https://doi.org/10.1111/j.1600-0714.2008.00659.x
Clark C, Shah S, Herman-Ferdinandez L, Ekshyyan O, Abreo F, Rong X, McLarty J, Lurie A, Milligan EJ, Nathan CO (2010) Teasing out the best molecular marker in the AKT/mTOR pathway in head and neck squamous cell cancer patients. Laryngoscope 120(6):1159–1165. https://doi.org/10.1002/lary.20917
Acknowledgements
We are thankful to the Fundação de Amparo a Pesquisa do Estado de Minas Gerais (APQ-01746-13) e o Conselho Nacional de Desenvolvimento Científico e Tecnológico (471130/2013-13) for the financial support. I would like to thank the AC Camargo Cancer Hospital (São Paulo, Brazil) for providing the Tissue Microarray Core facility and Mr. Carlos Ferreira Nascimento for the technical support.
Funding sources
This study was supported by the Fundação de Amparo a Pesquisa do Estado de Minas Gerais (grant number APQ-01746-13) e o Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant number 471130/2013-13), Brazil.
Author information
Authors and Affiliations
Contributions
Flávia Sayuri Matsuo and Marilia Ferreira Andrade performed all experiments and wrote the manuscript; Adriano Mota Loyola did the histopathological review of all lesions and oral healthy mucosa, interpreted the immunohistochemical stainings, and revised the manuscript; Sindeval Jose Silva raised patients’ medical files and revised the manuscript; Marcelo José Barbosa Silva and Sérgio Vitorino Cardoso performed all statistical analyses, interpreted data, and revised the manuscript; Paulo Rogério de Faria contributed to the design and data analyses, and wrote the manuscript. All authors approved the final manuscript prior to its submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of ethical approval
This study was approved by the Committee on Research and Ethics of the Federal University of Uberlândia (CAAE number: 15188713.9.0000.5152).
Electronic supplementary material
ESM 1
(DOCX 12923 kb)
Rights and permissions
About this article
Cite this article
Matsuo, F.S., Andrade, M.F., Loyola, A.M. et al. Pathologic significance of AKT, mTOR, and GSK3β proteins in oral squamous cell carcinoma-affected patients. Virchows Arch 472, 983–997 (2018). https://doi.org/10.1007/s00428-018-2318-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2318-0