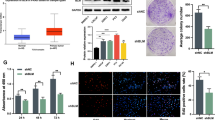
Abstract
Both plakophilins (PKP) 1 and 3 play a role in the progression of prostate cancer. The RNA-binding proteins (RBPs) GAP-SH3-binding protein (G3BP), fragile-X-related protein 1 (FXR1), poly(A)-binding protein, cytoplasmic 1 (PABPC1), and up-frameshift factor 1 (UPF1) are associated with PKP3. All these RBPs have an impact on RNA metabolism. Until recently, the PKP-associated RBPs have not been analyzed in prostate cancer. In the current study, we showed by affinity purification that the PKP3-associated RBPs were also binding partners of PKP1. We examined the expression of PKP1/3-associated RBPs and PKP1/3 in prostate cell lines, tumor-free prostate, and 136 prostatic adenocarcinomas by immunofluorescence and immunoblot. All four RBPs G3BP, FXR1, UPF1, and PABPC1 were expressed in the glandular epithelium of the normal prostate. PKP1 and FXR1 were strongly reduced in tumor tissues with Gleason score >7 and diminished expression of PKP1 and FXR1 also appeared to be associated with a metastatic phenotype. Additionally, the predominant nuclear localization of UPF1 in normal glandular cells and low grade tumors was switched to a more cytoplasmic pattern in carcinomas with Gleason score >7. Our findings suggest that PKP1 and FXR1 may have a tumor-suppressive function and are downregulated in more aggressive tumors. Collectively, PKP1/3-associated RBPs FXR1 and UPF1 may have a functional role in prostate cancer progression and metastasis and highlight the potential importance of posttranscriptional regulation of gene expression and nonsense-mediated decay in cancer.






Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Jackson RJ, Hellen CU, Pestova TV (2008) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 11(2):113–127
Glisovic T, Bachorik JL, Yong J, Dreyfuss G (2008) RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett 582(14):1977–1986
Jeanes A, Gottardi CJ, Yap AS (2008) Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene 27(55):6920–6929
Vasioukhin V, Fuchs E (2001) Actin dynamics and cell–cell adhesion in epithelia. Curr Opin Cell Biol 13(1):76–84
Bass-Zubek AE, Godsel LM, Delmar M, Green KJ (2009) Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr Opin Cell Biol 21(5):708–716
Neuber S, Muhmer M, Wratten D, Koch PJ, Moll R, Schmidt A (2010) The desmosomal plaque proteins of the plakophilin family. Dermatol Res Pract 2010:101452
Breuninger S, Reidenbach S, Sauer CG, Strobel P, Pfitzenmaier J, Trojan L, Hofmann I (2010) Desmosomal plakophilins in the prostate and prostatic adenocarcinomas: implications for diagnosis and tumor progression. Am J Pathol 176(5):2509–2519
Valenta T, Hausmann G, Basler K (2012) The many faces and functions of beta-catenin. EMBO J 31(12):2714–2736
Hofmann I, Casella M, Schnolzer M, Schlechter T, Spring H, Franke WW (2006) Identification of the junctional plaque protein plakophilin 3 in cytoplasmic particles containing RNA-binding proteins and the recruitment of plakophilins 1 and 3 to stress granules. Mol Biol Cell 17(3):1388–1398
Irvine K, Stirling R, Hume D, Kennedy D (2004) Rasputin, more promiscuous than ever: a review of G3BP. Int J Dev Biol 48(10):1065–1077
Vasudevan S, Steitz JA (2007) AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell 128(6):1105–1118
Siomi MC, Siomi H, Sauer WH, Srinivasan S, Nussbaum RL, Dreyfuss G (1995) FXR1, an autosomal homolog of the fragile X mental retardation gene. EMBO J 14(11):2401–2408
Khera TK, Dick AD, Nicholson LB (2010) Fragile X-related protein FXR1 controls post-transcriptional suppression of lipopolysaccharide-induced tumour necrosis factor-alpha production by transforming growth factor-beta1. FEBS J 277(13):2754–2765
Gorgoni B, Richardson WA, Burgess HM, Anderson RC, Wilkie GS, Gautier P, Martins JP, Brook M, Sheets MD, Gray NK (2011) Poly(A)-binding proteins are functionally distinct and have essential roles during vertebrate development. Proc Natl Acad Sci U S A 108(19):7844–7849
Brook M, Gray NK (2012) The role of mammalian poly(A)-binding proteins in co-ordinating mRNA turnover. Biochem Soc Trans 40(4):856–864
Mangus DA, Evans MC, Jacobson A (2003) Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol 4(7):223
Amrani N, Ghosh S, Mangus DA, Jacobson A (2008) Translation factors promote the formation of two states of the closed-loop mRNP. Nature 453(7199):1276–1280
Cosson B, Berkova N, Couturier A, Chabelskaya S, Philippe M, Zhouravleva G (2002) Poly(A)-binding protein and eRF3 are associated in vivo in human and Xenopus cells. Biol Cell 94(4–5):205–216
Ivanov PV, Gehring NH, Kunz JB, Hentze MW, Kulozik AE (2008) Interactions between UPF1, eRFs, PABP and the exon junction complex suggest an integrated model for mammalian NMD pathways. EMBO J 27(5):736–747
Nicholson P, Yepiskoposyan H, Metze S, Zamudio Orozco R, Kleinschmidt N, Muhlemann O (2010) Nonsense-mediated mRNA decay in human cells: mechanistic insights, functions beyond quality control and the double-life of NMD factors. Cell Mol Life Sci 67(5):677–700
Czaplinski K, Weng Y, Hagan KW, Peltz SW (1995) Purification and characterization of the Upf1 protein: a factor involved in translation and mRNA degradation. RNA 1(6):610–623
Lukong KE, Chang KW, Khandjian EW, Richard S (2008) RNA-binding proteins in human genetic disease. Trends Genet 24(8):416–425
Silvera D, Formenti SC, Schneider RJ (2010) Translational control in cancer. Nat Rev Cancer 10(4):254–266
Wurth L (2012) Versatility of RNA-binding proteins in cancer. Comp Funct Genom 2012:178525
Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, Hochman T, Formenti SC, Schneider RJ (2009) Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol 11(7):903–908
Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Teruya-Feldstein J, Pelletier J, Lowe SW (2007) Dissecting eIF4E action in tumorigenesis. Genes Dev 21(24):3232–3237
Abdelmohsen K, Gorospe M (2010) Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip Rev RNA 1(2):214–229
Hinman MN, Lou H (2008) Diverse molecular functions of Hu proteins. Cell Mol Life Sci 65(20):3168–3181
Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL (2005) The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 29(9):1228–1242
Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P (1995) Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. Vitro Cell Dev Biol Anim 31(1):14–24
Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF (1978) Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer 21(3):274–281
Hofmann I, Kuhn C, Franke WW (2008) Protein p0071, a major plaque protein of non-desmosomal adhering junctions, is a selective cell-type marker. Cell Tissue Res 334(3):381–399
Kirkpatrick LL, McIlwain KA, Nelson DL (1999) Alternative splicing in the murine and human FXR1 genes. Genomics 59(2):193–202
Dube M, Huot ME, Khandjian EW (2000) Muscle specific fragile X related protein 1 isoforms are sequestered in the nucleus of undifferentiated myoblast. BMC Genet 1:4
Schmidt A, Langbein L, Rode M, Pratzel S, Zimbelmann R, Franke WW (1997) Plakophilins 1a and 1b: widespread nuclear proteins recruited in specific epithelial cells as desmosomal plaque components. Cell Tissue Res 290(3):481–499
Atkin AL, Altamura N, Leeds P, Culbertson MR (1995) The majority of yeast UPF1 co-localizes with polyribosomes in the cytoplasm. Mol Biol Cell 6(5):611–625
Singh G, Jakob S, Kleedehn MG, Lykke-Andersen J (2007) Communication with the exon-junction complex and activation of nonsense-mediated decay by human Upf proteins occur in the cytoplasm. Mol Cell 27(5):780–792
Thomas MG, Martinez Tosar LJ, Loschi M, Pasquini JM, Correale J, Kindler S, Boccaccio GL (2005) Staufen recruitment into stress granules does not affect early mRNA transport in oligodendrocytes. Mol Biol Cell 16(1):405–420
Buchan JR, Parker R (2009) Eukaryotic stress granules: the ins and outs of translation. Mol Cell 36(6):932–941
Anderson P, Kedersha N (2008) Stress granules: the Tao of RNA triage. Trends Biochem Sci 33(3):141–150
Sobolik-Delmaire T, Katafiasz D, Keim SA, Mahoney MG, Wahl JK 3rd (2007) Decreased plakophilin-1 expression promotes increased motility in head and neck squamous cell carcinoma cells. Cell Commun Adhes 14(2–3):99–109
Papagerakis S, Shabana AH, Depondt J, Gehanno P, Forest N (2003) Immunohistochemical localization of plakophilins (PKP1, PKP2, PKP3, and p0071) in primary oropharyngeal tumors: correlation with clinical parameters. Hum Pathol 34(6):565–572
Kaz AM, Luo Y, Dzieciatkowski S, Chak A, Willis JE, Upton MP, Leidner RS, Grady WM (2012) Aberrantly methylated PKP1 in the progression of Barrett’s esophagus to esophageal adenocarcinoma. Gene Chromosome Cancer 51(4):384–393
Schwarz J, Ayim A, Schmidt A, Jager S, Koch S, Baumann R, Dunne AA, Moll R (2006) Differential expression of desmosomal plakophilins in various types of carcinomas: correlation with cell type and differentiation. Hum Pathol 37(5):613–622
Moll I, Kurzen H, Langbein L, Franke WW (1997) The distribution of the desmosomal protein, plakophilin 1, in human skin and skin tumors. J Invest Dermatol 108(2):139–146
Furukawa C, Daigo Y, Ishikawa N, Kato T, Ito T, Tsuchiya E, Sone S, Nakamura Y (2005) Plakophilin 3 oncogene as prognostic marker and therapeutic target for lung cancer. Cancer Res 65(16):7102–7110
Aigner K, Descovich L, Mikula M, Sultan A, Dampier B, Bonne S, van Roy F, Mikulits W, Schreiber M, Brabletz T, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A (2007) The transcription factor ZEB1 (deltaEF1) represses plakophilin 3 during human cancer progression. FEBS Lett 581(8):1617–1624
Valladares-Ayerbes M, Diaz-Prado S, Reboredo M, Medina V, Lorenzo-Patino MJ, Iglesias-Diaz P, Haz M, Pertega S, Santamarina I, Blanco M, Quindos-Varela M, Figueroa A, Anton-Aparicio LM (2010) Evaluation of plakophilin-3 mRNA as a biomarker for detection of circulating tumor cells in gastrointestinal cancer patients. Cancer Epidemiol Biomarkers Prev 19(6):1432–1440
Demirag GG, Sullu Y, Gurgenyatagi D, Okumus NO, Yucel I (2011) Expression of plakophilins (PKP1, PKP2, and PKP3) in gastric cancers. Diagn Pathol 6:1
Demirag GG, Sullu Y, Yucel I (2012) Expression of plakophilins (PKP1, PKP2, and PKP3) in breast cancers. Med Oncol 29(3):1518–1522
Takahashi H, Nakatsuji H, Takahashi M, Avirmed S, Fukawa T, Takemura M, Fukumori T, Kanayama H (2012) Up-regulation of plakophilin-2 and down-regulation of plakophilin-3 are correlated with invasiveness in bladder cancer. Urology 79(1):240.e1–240.e8
Parker F, Maurier F, Delumeau I, Duchesne M, Faucher D, Debussche L, Dugue A, Schweighoffer F, Tocque B (1996) A Ras-GTPase-activating protein SH3-domain-binding protein. Mol Cell Biol 16(6):2561–2569
Gorlach M, Burd CG, Dreyfuss G (1994) The mRNA poly(A)-binding protein: localization, abundance, and RNA-binding specificity. Exp Cell Res 211(2):400–407
Kennedy D, French J, Guitard E, Ru K, Tocque B, Mattick J (2001) Characterization of G3BPs: tissue specific expression, chromosomal localisation and rasGAP(120) binding studies. J Cell Biochem 84(1):173–187
Siomi MC, Zhang Y, Siomi H, Dreyfuss G (1996) Specific sequences in the fragile X syndrome protein FMR1 and the FXR proteins mediate their binding to 60S ribosomal subunits and the interactions among them. Mol Cell Biol 16(7):3825–3832
Garnon J, Lachance C, Di Marco S, Hel Z, Marion D, Ruiz MC, Newkirk MM, Khandjian EW, Radzioch D (2005) Fragile X-related protein FXR1P regulates proinflammatory cytokine tumor necrosis factor expression at the post-transcriptional level. J Biol Chem 280(7):5750–5763
Caudy AA, Myers M, Hannon GJ, Hammond SM (2002) Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev 16(19):2491–2496
Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Nelson DL, Moses K, Warren ST (2004) Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci 7(2):113–117
Mortensen RD, Serra M, Steitz JA, Vasudevan S (2011) Posttranscriptional activation of gene expression in Xenopus laevis oocytes by microRNA-protein complexes (microRNPs). Proc Natl Acad Sci U S A 108(20):8281–8286
Xu XL, Zong R, Li Z, Biswas MH, Fang Z, Nelson DL, Gao FB (2011) FXR1P but not FMRP regulates the levels of mammalian brain-specific microRNA-9 and microRNA-124. J Neurosci 31(39):13705–13709
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Disc 2(5):401–404
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL (2010) Integrative genomic profiling of human prostate cancer. Cancer Cell 18(1):11–22
Comtesse N, Keller A, Diesinger I, Bauer C, Kayser K, Huwer H, Lenhof HP, Meese E (2007) Frequent overexpression of the genes FXR1, CLAPM1 and EIF4G located on amplicon 3q26–27 in squamous cell carcinoma of the lung. Int J Cancer 120(12):2538–2544
Lykke-Andersen J, Shu MD, Steitz JA (2000) Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell 103(7):1121–1131
Mendell JT, ap Rhys CM, Dietz HC (2002) Separable roles for rent1/hUpf1 in altered splicing and decay of nonsense transcripts. Science 298(5592):419–422
Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J (2007) Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318(5851):798–801
Chawla R, Redon S, Raftopoulou C, Wischnewski H, Gagos S, Azzalin CM (2011) Human UPF1 interacts with TPP1 and telomerase and sustains telomere leading-strand replication. EMBO J 30(19):4047–4058
Perrin-Vidoz L, Sinilnikova OM, Stoppa-Lyonnet D, Lenoir GM, Mazoyer S (2002) The nonsense-mediated mRNA decay pathway triggers degradation of most BRCA1 mRNAs bearing premature termination codons. Hum Mol Genet 11(23):2805–2814
Gardner LB (2010) Nonsense-mediated RNA decay regulation by cellular stress: implications for tumorigenesis. Mol Cancer Res 8(3):295–308
Wang D, Zavadil J, Martin L, Parisi F, Friedman E, Levy D, Harding H, Ron D, Gardner LB (2011) Inhibition of nonsense-mediated RNA decay by the tumor microenvironment promotes tumorigenesis. Mol Cell Biol 31(17):3670–3680
Acknowledgments
The authors thank Dr. Tim Holland-Letz (Biostatistics, DKFZ, Heidelberg, Germany) for advice in statistical analysis. This study was supported by grants from the German Cancer Aid and the “Deutsche Forschungsgemeinschaft” (STR 638/2-1). Cheng Yang acknowledges a fellowship from the Chinese Scholarship Council.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supple Fig. 1
Double immunofluorescence of PKP1, symplekin, and UPF1 using two different UPF1 antibodies in BPH-1 cells (UPF1-ab1, antibody from Bethyl Laboratories; UPF2-ab2, antibody from Sigma-Aldrich). Localization of PKP1 and symplekin is shown in the red channel (a, b, c). Occurrence of the UPF1 (a’, b’, c’) is shown in the green channel. Merged pictures are also presented (a”, c”, c”). Cells were fixed with formaldehyde. Scale bar, 20 μm (JPEG 174 kb)
Supple Fig. 2
Detection of G3BP on formaldehyde-fixed, paraffin-embedded tissues of tumor-free human prostate (a) compared with the localization of keratin 14 (a’). The merged picture is given in (a”). Although G3BP is detectable in both luminal and basal cells, basal cells show a stronger G3BP expression. Scale bar, 20 μm (JPEG 30 kb)
Rights and permissions
About this article
Cite this article
Yang, C., Ströbel, P., Marx, A. et al. Plakophilin-associated RNA-binding proteins in prostate cancer and their implications in tumor progression and metastasis. Virchows Arch 463, 379–390 (2013). https://doi.org/10.1007/s00428-013-1452-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-013-1452-y