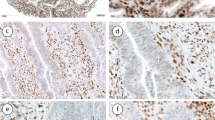
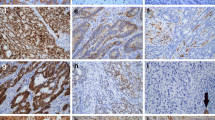
Abstract
Based on the principle of nonsense-mediated mRNA decay, we sought to identify MLH1 or MSH2-deficient colorectal tumours through relative quantification of mRNA expression with real-time PCR (RT-PCR) analysis. MLH1 and MSH2 mRNAs were almost equally expressed as defined by MLH1 to MSH2 transcript ratio (mean 1.41) in microsatellite stable, mismatch repair (MMR) proficient tumours (n = 16). A close correlation between loss of protein expression and MMR–mRNA levels was found in highly microsatellite instable (MSI-H) tumours deficient of MLH1 or MSH2. MLH1/MSH2 ratio was low in 11 sporadic and nine hereditary MLH1-deficient carcinomas (mean 0.51), whereas the ratio was high in 17 MSH2-deficient hereditary non-polyposis colorectal cancer (HNPCC) associated carcinomas (mean 6.8). Notably, in the normal tissues of HNPCC patients with MSH2 mutations, the MLH1/MSH2 transcript ratios were significantly elevated (ratio > 2.0) as compared to the ratios of normal mucosa in patients with MMR-proficient tumours (27 of 32 ratio < 2.0; p = 0.00113). Analysis of B-lymphocytes of HNPCC patients with proven MMR gene mutation confirmed these findings. In conclusion, RT-PCR allows relative quantification of MMR gene mRNA expression in formalin-fixed and paraffin-embedded tissue. Furthermore, this approach enables quantification of haploinsufficiency due to nonsense-mediated mRNA decay in normal tissue and B-lymphocytes from patients carrying MSH2 germline mutations and may be useful for identification of asymptomatic carriers of pathogenic germline mutations.


Similar content being viewed by others
References
Aaltonen LA, Salovaara R, Kristo P, Canzian F, Hemminki A, Peltomäki P, Chadwick RB, Kääriäinen H, Eskelinen M, Järvinen H, Mecklin JP, Chapelle de la (1998) Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med 338(21):1481–1487
Aarnio M, Sankila R, Pukkala E, Aaltonen LA, de la Chapelle A, Peltomäki P, Mecklin JP, Jarvin HJ (1998) Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 81(2):214–218
Abu-Safieh L, Vithana EN, Mantel I, Holder GE, Pelosini L, Bird AC, Bhattacharya SS (2006) A large deletion in the adRP gene PRPF31: evidence that haploinsufficiency is the cause of disease. Mol Vis 18:384–388
Bai AH, To KF, Chan WF, Man EP, Lo KW, Lee JF, Sung JJ, Leung WK (2004) Promotor hypermethylation of tumor-related genes in the progression of colorectal neoplasia. Int J Cancer 10:846–853
Brinda RS, Glazer PM (2007) Co-repression of mismatch repair gene expression by hypoxia in cancer cells: role of the Myc/Max network. Cancer Lett 252:93–103
Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Rüschoff J (1997) Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res 57:4749–4756
Durand-Faucher K, Rabinovitch-Chable H, Dzugan H, Charret S, Aubry K, Genet D, Léobon S, Tubiana-Mathieu N, Cook-Moreau J, Rigaud M, Sturtz FG (2005) A quantitative RT-PCR method to determine topoisomerase I mRNA levels in human tissue samples. Clin Chem Lab 43:707–714
Evans DG, Walsh S, Jeacock J (1997) Incidence of hereditary non-polyposis colorectal cancer in a population-based study of 1137 consecutive cases of colorectal cancer. Br J Surg 84(9):1281–1285
Gryfe R, Gallinger S (2001) Microsatellite instability, mismatch repair deficiency, and colorectal cancer. Surgery 130:17–20
Hentze MW, Kulozik AE (1999) A perfect message: RNA surveillance and nonsense-mediated decay. Cell 5:3007–3010
Holmberg M, Kristo P, Chadwicks RB, Mecklin JP, Jarvinen H, de la Chapelle A, Nystrom-Lahti M, Peltomaki P (1998) Mutation sharing, predominant involvement of the MLH1 gene and description of four novel mutations in hereditary nonpolyposis colorectal cancer. Mutations in brief no. 144. Online.. Hum Mutat 11(6):482–486
Jass JR (2000) hMLH1 and hMSH2 immunostaining in colorectal cancer. Gut 47:315–316
Laimer M, Klausegger A, Aberer W et al (2006) Haploinsufficiency due to deletion within the 3′-UTR of C1-IHN-gene associated with hereditary angioedema. Gent Med 8:249–254
Lamberti C, Kruse R, Ruelfs C, Caspari R, Wang Y, Jungck M, Mathiak M, Malayeri HR, Friedl W, Sauerbruch T, Propping P (1999) Microsatellite instability—a useful diagnostic tool to select patients at high risk for hereditary non-polyposis colorectal cancer: a study in different groups of patients with colorectal cancer. Gut 44:839–843
Lanza G, Gafà R, Maestri I, Santini A, Matteuzzi M, Cavazzini L (2002) Immunohistochemical pattern of MLH1/MSH2 expression is related to clinical and pathological features in colorectal adenocarcinomas with microsatellite instability. Mod Pathol 15:741–749
Linnebacher M, Gebert J, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M (2004) Frameshift peptode-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer 2001; 93:6–11
Liu Q, Jiang L, Liu WL, Kang XJ, Ao Y, Sun M, Luo Y, Song Y, Lo WH, Zhang X (2006) Two novel mutations and evidence for haploinsufficiency of the ADAR gene in dyschromatosis symmetrica hereditaria. Br J Dermatol 154:636–642
Loukola A, Eklin K, Laiho P, Salovaara R, Kristo P, Jarvinen H, Mecklin JP, Launonen V, Aaltonen LA (2001) Microsatellite marker analysis in screening for hereditary nonpolyposis colorectal cancer (HNPCC). Cancer Res 61:4545–4549
Lynch HT, de la Chapelle A (1999) Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet 36:801–818
Lynch HT, Chapelle de la A (2003) Hereditary colorectal cancer. N Engl J Med 2 348:919–932
Mangold E, Pagenstecher C, Friedl W, The German HNPCC Consortium (2005) Spectrum and frequencies of mutations in MSH2 and MLH1 identified in 1721 German families suspected of hereditary nonpolyposis colorectal cancer. Int J Cancer 2005:692–702
Müller W, Burgart LJ, Krause-Paulus R, Thibodeau SN, Almeida M, Bocker Edmonston T, Boland CR, Sutter C, Jass JR, Lindblom A, Lubinski J, MacDermot K, Sanders DSA, Morreau H, Müller A, Oliani C, Orntoft T, Ponz De Leon M, Rosty C, Rodriguez-Bigas M, Rüschoff J, Ruszkiewicz A, Sabourin J, Salovaara R, Möslein G, the ICG-HNPCC (2001) The reliability of immunohistochemistry as a prescreening method for the diagnosis of hereditary nonpolyposis colorectal cancer (HNPCC)—results of an international collaborative study. Familial Cancer 1:87–92
Müller A, Guiffre G, Bocker Edmonston T, Mathiak M, Roggendorf B, Heinmöller E, Brodegger T, Tuccari G, Mangold E, Buettner R, Rüschoff J (2004) Challenges and pitfalls in HNPCC screening by microsatellite analysis and immunohistochemistry. J Mol Diag 6:308–315
Nuovo GJ, Nakagawa H, Sotamaa K et al (2006) Hypermethylation of the MLH1 promotor with concomitant absence of transcript and protein occurs in small patches of crypt cells in unaffected mucosa from sporadic colorectal carcinoma. Diag Mol Path 15:17–23
Plaschke J, Krüger ST, Pistorius ST, Theissig F, Saeger HD, Schackert HK (2002) Involvement of hMSH6 in the development of hereditary and sporadic colorectal cancer revealed by immunostaining is based on germline mutations, but rarely on somatic inactivation. Int J Cancer 97:643–648
Peltomäki P (2001) Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum Mol Genet 10:735–740
Rigau V, Sebbagh N, Olschwang S, Paraf F, Mourra N, Parc Y, Flejou JF (2003) Microsatellite instability in colorectal carcinoma. The comparison of immunohistochemistry and molecular biology suggests a role for hMSH6 immunostaining. Arch Pathol Lab Med 127:694–700
Rüschoff J, Bocker T, Schlegel J, Stamm G, Hofstaedter F (1995) Microsatellite instability: new aspects in the carcinogenesis of colorectal carcinoma. Virchow Arch 426:215–222
Salovaara R, Loukola A, Kristo P, Kääriäinen H, Ahtola H, Eskelinen M, Härkönen N, Julkunen R, Kangas E, Ojala S, Tulikoura J, Valkamo E, Järvinen H, Mecjklin JP, Aaltonen LA, Chapelle de la A (2000) Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol 11:2193–2200
Thibodeau SN, French AJ, Roche PC, Roche PC, Cunningham JM, Tester DJ, Lindor NM, Moslein G, Baker SM, Liskay RM, Burgart LJ, Honchel R, Halling KC (1996) Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res 56:4836–4840
Tournier I, Raux G, Di Fiore F, Marechal I, Leclerc C, Martin C, Wang Q, Buisine MP, Stoppa-Lyonnet D, Olschwang S, Frebourg T, Tosi M (2004) Analysis of the allele-specific expression of the mismatch repair gene MLH1 using a simple DHPLC-based method. Hum Mutat 23(4):379–384
Acknowledgements
The work of the Consortium is supported by a multi-center grant from the Deutsche Krebshilfe (German Cancer Aid), Bonn, Germany. This study is in part the diploma thesis of D.Z. We thank Katja Bräutigam and Katja Goldacker for excellent technical assistance.
This work is also supported by the DFG-Grand 1304 (Klinische Forschergruppe 179)
Conflict of interest statement
We declare that we have no conflict of interest
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Members of the German HNPCC Consortium are listed in the Appendix.
Appendix
Appendix
The German HNPCC Consortium consists of the following centers (in alphabetic order): Bochum (K Schulmann, FE Brasch, JT Epplen, KM Müller, C Pox, S Stemmler, J Willert), Bonn (in addition to authors: W Friedl, J Girmscheid, A Hirner, C Lamberti, M Mathiak, P Propping, T Sauerbruch, K Siberg), Düsseldorf (G Moeslein, T Goecke, A Hansmann, S Höwer, C Poremba, A Unger, C Wieland), Dresden (in addition to authors: D Aust, F Balck, R Höhl, S Krüger, E Schröck, S Pistorius), Heidelberg (in addition to authors: F Cremer, J Gebert, M Keller, P Kienle, M Kloor, U Mazitschek, M Tariverdian), München/Regensburg (in addition to authors: I Becker, E Holinski-Feder, G Keller, R Kopp, Y Müller-Koch, K Ott, P Rümmele), center for reference pathology Kassel and center for documentation and biometry in Leipzig (in addition to authors: J Forberg, M Herold, M Loeffler, J Schaefer, R Speer).
Rights and permissions
About this article
Cite this article
Müller, A., Zielinski, D., Friedrichs, N. et al. Reduced mRNA expression in paraffin-embedded tissue identifies MLH1- and MSH2-deficient colorectal tumours and potential mutation carriers. Virchows Arch 453, 9–16 (2008). https://doi.org/10.1007/s00428-008-0637-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-008-0637-2