Abstract
Bilateria encompass the vast majority of the animal phyla. As the name states, they are bilaterally symmetric, that is with a morphologically clear main body axis connecting their anterior and posterior ends, a second axis running between their dorsal and ventral surfaces, and with a left side being roughly a mirror image of their right side. Bone morphogenetic protein (BMP) signalling has widely conserved functions in the formation and patterning of the second, dorso-ventral (DV) body axis, albeit to different extents in different bilaterian species. Whilst initial findings in the fruit fly Drosophila and the frog Xenopus highlighted similarities amongst these evolutionarily very distant species, more recent analyses featuring other models revealed considerable diversity in the mechanisms underlying dorsoventral patterning. In fact, as phylogenetic sampling becomes broader, we find that this axis patterning system is so evolvable that even its core components can be deployed differently or lost in different model organisms. In this review, we will try to highlight the diversity of ways by which BMP signalling controls bilaterality in different animals, some of which do not belong to Bilateria. Future research combining functional analyses and modelling is bound to give us some understanding as to where the limits to the extent of the evolvability of BMP-dependent axial patterning may lie.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Brief overview of the BMP signalling pathway
BMPs are secreted signalling proteins and members of the transforming growth factor-β (TGF-β) superfamily. The TGF-β signalling pathway is one of the evolutionary novelties of animals (Babonis and Martindale 2017; Huminiecki et al. 2009). Mature BMP ligands are produced from pro-proteins by proteolytic processing and act as homo- or heterodimers, with heterodimers tending to be the “stronger” signals (Aono et al. 1995; Bauer et al. 2023; Cui et al. 2001; Fritsch et al. 2012; Hazama et al. 1995; Künnapuu et al. 2009, 2014; Little and Mullins 2009; Nishimatsu and Thomsen 1998; Shimmi et al. 2005; Sopory et al. 2010; Suzuki et al. 1997; Tajer et al. 2021). The “main” BMP ligands belong to the BMP2/4 and BMP5-8 families, however, other BMPs, e.g. ADMP and GDF5-6, exist and play a role in DV patterning (Genikhovich et al. 2015; Lapraz et al. 2015; Reversade and De Robertis 2005). BMP dimers bind and activate heterotetrameric BMP receptor complexes, consisting of two type I and two type II receptors (reviewed in Heldin and Moustakas (2016)). Receptor complex activation triggers the phosphorylation of the receptor-regulated Smads (R-Smads), Smad1/5Footnote 1 (Mad in DrosophilaFootnote 2) - transcriptional effectors that form a heterotrimeric complex with a so-called common-partner Smad (Co-Smad), Smad4 (Medea in Drosophila) and translocate into the nucleus, where they activate or repress gene expression assisted by a range of co-factors (reviewed in Hill (2016); Fig. 1A). Inhibition of BMP signalling is possible at different steps along the signalling pathway. For example, inside the cell, the R-Smad/Co-Smad complex action is counteracted by inhibitory Smads (I-Smads), Smad6 and Smad7, which inhibit R-Smad phosphorylation by interacting with the type I receptor, as well as by several other mechanisms (Miyazawa and Miyazono 2017), whilst at the cell surface, a non-functional type I BMP receptor BAMBI (BMP and Activin membrane-bound inhibitor) interferes with the assembly of active TGF-β receptor complexes (Onichtchouk et al. 1999). However, the already quite complex intracellular regulation of BMP signalling is easily eclipsed by the mind-boggling complexity of the BMP signalling regulation by extracellular proteins. Chordin, Noggin, Follistatin, Gremlin and Cerberus are extracellular BMP signalling inhibitors whose function is at least partially redundant (Bachiller et al. 2000; Brazil et al. 2015; Dal-Pra et al. 2006; Francois et al. 1994; Genikhovich et al. 2015; Holley et al. 1996; Iemura et al. 1998; Miller et al. 2019; Piccolo et al. 1999; Sasai et al. 1994; Tajer et al. 2021; Zimmerman et al. 1996). Amongst them, Chordin (Short gastrulation, Sog in Drosophila) stands out as a molecule with a dual anti-BMP and pro-BMP function. Similar to other inhibitors, Chordin binds BMP dimers preventing them from binding the receptors. On the other hand, Chordin-BMP complexes are capable of diffusion and, upon cleavage of Chordin by the metalloproteases Tolloid or BMP1, release BMP dimers promoting signalling at a distance from the Chordin source (Marqués et al. 1997; Piccolo et al. 1997; Scott et al. 1999; Shimmi et al. 2005; Tuazon et al. 2020; Wang and Ferguson 2005). Many other extracellular regulators of BMP signalling are known, especially in chordates: twisted gastrulation (Tsg) with its complex pro- and anti-BMP activities, a Tolloid antagonist Sizzled, a Tolloid agonist Ont1, another Tolloid inhibitor Crescent, an ADMP regulator Pinhead, and more (Chang et al. 2001; Dal-Pra et al. 2006; Imai et al. 2012; Inomata et al. 2008; Itoh et al. 2021; Lee et al. 2006; Oelgeschlager et al. 2000; Peluso et al. 2011; Ploper et al. 2011; Ross et al. 2001; Scott et al. 2001; Shimmi and O'Connor 2003; Wang and Ferguson 2005; Yan et al. 2019).
BMP signalling. A Illustration of the core BMP signalling components. BMP dimers are bound and inhibited by Chordin, which blocks them from interacting with the BMP receptor complex (left). In the absence of Chordin, or after Tolloid-catalysed cleavage of Chordin and release of the BMP ligand, BMPs activate the receptor complex leading to phosphorylation of Smad1/5 (indicated by pink colour). Phosphorylated Smad1/5, together with the Co-Smad Smad4, enters the nucleus and regulates transcription of BMP target genes (right). B BMP-mediated patterning according to the shuttling model: close to the Chordin source, a high concentration of Chordin blocks BMPs from binding their receptors. The BMP-Chordin complex is diffusible and can effectively transport BMPs. Only at a distance of the Chordin source, release of BMPs from the inhibitory complex after Tolloid cleavage enables BMPs to bind receptors rather than another, yet uncleaved, Chordin molecule. In the shuttling model, BMP signalling is activated at a distance from the Chordin source, independent of the BMP source location. BMP protein concentration profile deliberately not shown since in the shuttling model it is irrelevant for the location of the BMP signalling domain (see Genikhovich et al. 2015). C BMP signalling in the source-sink model: BMP and Chordin source oppose each other. BMPs form a concentration gradient from their source and activate receptor complexes where they can bind them. Chordin forms an opposing gradient and BMP signalling is inhibited where Chordin concentration is high. Chordin acts locally to inhibit BMP signalling and does neither to inhibit, nor promote BMP signalling at a distance. Pink colour indicates phosphorylated Smad1/5 (active BMP signalling) in all panels
Diverse mechanisms establish BMP signalling gradients along the DV axis
BMPs in the Drosophila embryo were amongst the first morphogens to be discovered (Ferguson and Anderson 1992a). The two BMP ligands responsible for the formation of the BMP signalling gradient along the second body axis are the dorsally produced BMP2/4 (Decapentaplegic, Dpp) and the ubiquitously produced BMP5-8 (Screw) (Arora et al. 1994; Jazwinska et al. 1999; Shimmi et al. 2005). BMP dimers form a BMP-Chordin-Tsg complex diffusing in the perivitelline space. BMP is released from this inhibitory complex by Tolloid-mediated cleavage of Chordin, allowing BMP to bind its receptors (Eldar et al. 2002; Marqués et al. 1997; Peluso et al. 2011; Ross et al. 2001; Shimmi and O'Connor 2003; Shimmi et al. 2005; Wang and Ferguson 2005). A Chordin-mediated flux of BMPs, BMP-dependent Chordin cleavage by Tolloid, and feedback regulation by BMP target genes together generate a steep dorsal-to-ventral gradient of nuclear pSmad1/5 (Ashe and Levine 1999; Eldar et al. 2002; Gavin-Smyth et al. 2013; Mizutani et al. 2005; Peluso et al. 2011; Srinivasan et al. 2002; Wang and Ferguson 2005). The mechanism of BMP gradient formation, which requires Chordin-assisted BMP transport away from the Chordin source and does not depend on the location of the BMP source, has been termed “BMP shuttling” (Fig. 1B). Experiments in frog and sea urchin embryos support a similar model (Ben-Zvi et al. 2008; Lapraz et al. 2009, 2015; Plouhinec et al. 2013).
However, shuttling and the pro-BMP function of Chordin is not conserved in all animals. In zebrafish, for example, loss of Chordin function does not reduce the maximum in the BMP signalling gradient (Pomreinke et al. 2017; Zinski et al. 2017). Instead of promoting the extracellular dispersion of BMP ligands and BMP signalling at a distance to the Chordin source, zebrafish Chordin appears to only repress BMP signalling and act as BMP sink in a “source-sink” mechanism. This was elegantly shown by using membrane-tethered Chordin: localized expression of this immobile Chordin can rescue chordin mutants, if the expression resembles the endogenous expression domain (Tuazon et al. 2020; Fig. 1C). There are also bilaterian clades in which Chordin was lost, and which use other means to pattern their DV axis, as we will describe below.
An overview of the BMP-dependent DV patterning across Bilateria
Now, after a brief summary of the main BMP signalling components and their modes of action underlying BMP-mediated patterning, let us have a look at the implementation of the BMP signalling for DV patterning in Bilateria (Fig. 2; the numbers at the branches correspond to the numbers in the text).
(1) Xenacoelomorpha
The phylogenetic position of Xenacoelomorpha is heavily disputed: currently, they are placed either as the earliest branching Bilateria, i.e. the sister group to “Nephrozoa”—a clade uniting deuterostomes and protostomes (Cannon et al. 2016), as a sister group to Ambulacraria (echinoderms and hemichordates) within Deuterostomia (Mulhair et al. 2022), or even as a sister group to Ambulacraria in a scenario where deuterostomes are considered paraphyletic (Kapli et al. 2021). In this paper, we will follow the views of Cannon et al. (2016); however, it is important to remember that the situation is far from resolved. Limited information on Xenacoelomorpha body axis patterning is available from studies of acoels. There is a complement of the main BMP signalling components including Chordin and three BMP ligands in Hofstenia miamia, including Hof-bmp5-8, a dorsally expressed Hof-bmp and a ventrally expressed Hof-admp.Footnote 3 However, the phylogenetic relationship of Hof-BMP to the BMP ligands of other animals is not entirely clear, and it is unlikely that Hof-BMP is a BMP2/4 orthologue. RNAi has been used to deplete two BMP ligands (Hof-bmp and Hof-admp) and Smads (Hof-smad1/5 and Hof-smad4) during regeneration, each condition leading to ventralisation (Srivastava et al. 2014). Moreover, bmp2/4 is expressed dorsally in the acoel Convolutriloba longifissura (Hejnol and Martindale 2008). Together, these findings indicate that BMP signalling in acoels is required for the formation of dorsal structures. The role of acoel Chordin is yet unknown.
Protostomia /Spiralia
Studies on various annelid, mollusc, brachiopod and planarian flatworm species are beginning to shed light on the function of BMP signalling in the early development of Lophotrochozoa/Spiralia. Although Chordin was lost in such established annelid models as the ragworm Platynereis and leech Helobdella, it is present in the early-branching annelid Owenia fusiformis, where an asymmetric expression along the dorsoventral axis has been reported (Martin-Zamora et al. 2023). The fact that BMP and Chordin are also implicated in DV patterning in brachiopods (Martin-Duran et al. 2016) and molluscs (Tan et al. 2022) indicates that BMP/Chordin-mediated dorsoventral patterning is an ancestral feature in Spiralia. However, DV patterning in Spiralia can be used as a perfect example of the extent of the developmental systems drift.
(2) Mollusca
In molluscs, BMP signalling was shown to have functions in secondary axis patterning in several cases, such as snails Ilyanassa (Lambert et al. 2016) and Lottia goshimai (Tan et al. 2022) where BMP2/4 depletion leads to at least a transient radialisation. The importance of Chordin in regulating DV patterning was demonstrated in Lottia, where asymmetric chordin expression is downstream of MAP kinase signalling from the organizer and required for asymmetric BMP signalling (Tan et al. 2022). Gene expression studies and BMP signalling inhibition indicate that BMP signalling similarly functions in DV axis formation in the oyster Crassostrea gigas (Tan et al. 2017, 2018). However, diversity in dorsoventral patterning exists also in molluscs; for example in the slipper snail Crepidula fornicata, BMP signalling was suggested not to impact DV patterning (Lyons et al. 2020).
(3) Brachiopoda
A study on axis formation in two brachiopod species, Novocrania anomala and Terebratalia transversa, reports that BMP signalling is active in the dorsal ectoderm, opposing the ventral chordin expression domain (Martin-Duran et al. 2016). Moreover, inhibition of BMP signalling results in ventralisation, but also anteriorization, which shows BMP-dependent secondary axis patterning in brachiopods and indicates that there is crosstalk between the dorsoventral and anterior–posterior patterning systems.
(4) Annelida
It was suggested that dorsoventral patterning could be fundamentally different between annelids and molluscs, since establishing the dorsoventral axis appears to be independent of BMP signalling in some annelid species such as Capitella teleta, where Activin/Nodal plays the central role in the DV patterning (Lanza and Seaver 2020; Webster et al. 2021). However, other annelids do use BMP signalling for DV patterning. The effect of the BMP signalling modulation in one of the most developed annelid models, Platynereis, suggests that dorsally expressed BMP2/4 is involved in DV patterning of the neurectoderm, and that the genes responding to this BMP modulation are the same in Platynereis, Drosophila and vertebrates (Denes et al. 2007). In the future, it will be interesting to find out in detail how Platynereis DV patterning is regulated, since Platynereis, like Capitella, does not have Chordin. In some cases, annelid BMP-dependent DV patterning can be quite bizarre. In the leech Helobdella, it acts in a curious cell-to-cell relay way. The DV patterning takes place within four pairs of “germinal bandlets” called n, o, p, and q, where n is the ventralmost and q is the dorsalmost bandlet. Two BMP2/4 and one ADMP gene are broadly expressed in all the bandlets, whilst BMP5-8 is only expressed in the dorsalmost q-bandlet and is the key BMP in this patterning process. BMP5-8 is necessary and sufficient to activate the expression of the BMP inhibitor Gremlin in the p-bandlet, which, in turn, is required for the specification of the o-bandlet, which is achieved by Gremlin apparently inhibiting BMP2/4-mediated but not BMP5-8-mediated signalling (Kuo and Weisblat 2011). In this context, it is important to repeat that chordin may be absent from many annelid genomes (including Platynereis and Capitella), but not from all. As already mentioned, the BMP-Chordin system appears to be involved in the DV patterning in the early-branching annelid Owenia (Martin-Zamora et al. 2023).
(5) Platyhelminthes
The function of BMP signalling in planarian flatworms has been studied mainly during regeneration of Dugesia japonica and Schmidtea mediterranea. Whilst it still remains to be shown by pSmad1/5 immunofluorescence where BMP signalling is active in the planarian body, RNAi of Smads (Smad1 and Smad4; Gavino and Reddien 2011; Reddien et al. 2007) indicates that it is required for the formation of dorsal structures. Consistent with this, RNAi of BMPs leads to ventralisation (Clark and Petersen 2023; Molina et al. 2007; Orii and Watanabe 2007; Reddien et al. 2007). Like in many other models, ventrally expressed ADMP acts in a feedback circuit with dorsal BMP4, probably to balance BMP signalling against fluctuations (Gavino and Reddien 2011; Molina et al. 2011). Intriguingly, a chordin gene has not been identified in planarians; however, Noggin and—unexpectedly, given the lack of Chordin—Tolloid homologs have been proposed to regulate BMP signalling in Schmidtea (Gavino and Reddien 2011; Molina et al. 2011; Reddien et al. 2007). In contrast, the function of noggin-like genes in Dugesia (Ogawa et al. 2002; Orii and Watanabe 2007) is not clear. Further research is necessary to understand the mechanism of the BMP-dependent DV patterning in flatworms, but this is hampered by the lack of a developed embryonic model.
Protostomia/Ecdysozoa
(6) Arthropoda
Work in different arthropods has provided fundamental insights into the functions of BMP signalling during development and the evolution of BMP-mediated patterning. Experiments in Drosophila melanogaster have been instrumental in our understanding of the BMP signalling pathway, from heterodimer formation and secretion (Bauer et al. 2023; Shimmi et al. 2005), to interactions with extracellular regulators (Peluso et al. 2011; Shimmi and O'Connor 2003), to extracellular transport and signalling (Simon et al. 2023), to the regulation of BMP target genes (Hoppe et al. 2020; Wharton et al. 2004). Nowadays, it is even possible to characterize the kinetics along the different steps of the signalling pathway (Romanova-Michaelides et al. 2022). Whilst the mechanisms underlying dorsoventral patterning discovered in Drosophila have been largely accepted as universal and even transferred to other model systems (BMP shuttling in frog, sea urchin), it is becoming clear that even within Arthropoda they are much more variable than initially expected.
BMP signalling is the strongest in the dorsal-most cells in the early Drosophila embryo (Mizutani et al. 2005; Peluso et al. 2011; Ross et al. 2001; Shimmi and O'Connor 2003). As we described above, the narrow domain of peak BMP signalling activity is explained by a shuttling mechanism that concentrates BMP ligands in the target domain located on the side of the embryo opposite to the Chordin source and is supported by transcription-based positive feedback (Eldar et al. 2002; Gavin-Smyth et al. 2013; Wang and Ferguson 2005). The detailed examination of DV patterning in Drosophila has revealed the intricate mechanisms regulating shuttling which involve not only Chordin and Tolloid, but also Tsg. Tsg can form a ternary complex with BMP and Chordin that efficiently inhibits BMP signalling and modulates Chordin cleavage (Eldar et al. 2002; Marqués et al. 1997; Peluso et al. 2011; Ross et al. 2001; Shimmi and O'Connor 2003; Shimmi et al. 2005; Wang and Ferguson 2005). Disruption of any of the shuttling components leads to DV patterning defects. Interestingly, Drosophila mutants for genes encoding BMP ligands are ventralised but do not display complete radialisation of the dorsoventral axis (Arora et al. 1994; Ferguson and Anderson 1992a, b). This is due to the determination of DV polarity upstream of BMP signalling in insects, where Toll/Dorsal signalling determines ventral cell fates (reviewed in Roth 2023).
However, flies are one of the younger insect clades. Moving from Drosophila towards insects in earlier branching groups has shown the decreasing importance of Toll signalling and the increasing role of BMP signalling in controlling the DV patterning in the flour beetle Tribolium castaneum (Nunes da Fonseca et al. 2008; van der Zee et al. 2006), the parasitoid wasp Nasonia vitripennis (Özüak et al. 2014) and the milkweed bug Oncopeltus fasciatus (Sachs et al. 2015). In Tribolium, pSmad1/5 is detected on the dorsal side of the embryo and requires the BMP2/4, Tc-dpp. This dorsal BMP signalling extends beyond the Tc-dpp expression domain and the dorsal restriction of signalling requires ventrally-expressed Chordin. This indicates that a shuttling-like system is in place (van der Zee et al. 2006). But, similar to Drosophila, Toll signalling is required in Tribolium to establish the DV axis (Nunes da Fonseca et al. 2008). In the wasp Nasonia, the influence of Toll signalling on DV patterning is reduced as embryos depleted of Toll retain DV polarity. Moreover, BMP2/4 (Nv-dpp) knockdown ventralises not only dorsal but also lateral tissue, revealing a stronger influence of BMP signalling on DV patterning than in Drosophila. Interestingly, DV patterning in the wasp appears to be independent of a Chordin, as no homolog was identified in Nasonia (Özüak et al. 2014). In the milkweed bug Oncopeltus, Chordin and Tsg are important for restricting BMP signalling to dorsal cells, allowing the formation of mesoderm at the opposing, ventral side. Chordin expression is negatively regulated by BMP signalling, as depletion of BMP2/4 or Tld ventralises the embryo and expands the chordin expression domain. Whilst Toll1 knockdown leads to a complete dorsalisation of the Oncopeltus embryo, knockdown of Chordin or BMP also abolishes DV patterning. It was proposed that Toll signalling is only required to provide initial DV asymmetry in Oncopeltus and that BMP signalling regulates the major part of DV patterning (Sachs et al. 2015). Counterintuitively, the DV patterning in the cricket Gryllus bimaculatus, which belongs to an earlier branching arthropod group than bugs, wasps and beetles, is very similar to Drosophila (Pechmann et al. 2021). Here, Toll is required to polarize the dorsoventral axis and Toll1 knockdown leads to the loss of ventral and lateral tissues. In contrast, depletion of BMP signalling components only abolishes dorsal structures and does not interfere with the mesoderm specification. Moreover, Chordin, which is crucial for dorsoventral patterning in Drosophila, is not present in Gryllus and other Gryllidae. However, given that in non-insect arthropods, such as the spider Achaearanea tepidariorum, both Chordin and BMP2/4 are required for proper dorsoventral patterning, it is clear that the cricket/fly similarity resulted from the independent loss of the leading role of BMP signalling in the DV patterning rather than represents an ancestral arthropod condition. In the spider embryo, chordin RNAi results in expanded BMP signalling and loss of ventral structures, whilst depletion of bmp2/4 transcripts leads to a reduction in dorsal tissues with an almost complete radialisation (Akiyama-Oda and Oda 2006).
Taken together, BMP-Chordin-based DV pattering was clearly ancestral for Arthropoda; however, this highly diverse animal group makes a prime example of how a patterning system can be independently modified and adjusted multiple times during evolution.
(7) Nematoda
In the nematode Caenorhabditis elegans, BMP signalling pathway components are present; however, they do not regulate embryonic dorsoventral patterning (Patterson and Padgett 2000). Nonetheless, they do have functions in later development and, potentially, patterning. For example, the BMP-like DBL-1 is important to regulate body size, and repression of BMP signalling involving DBL-1 is required for the dorsoventral patterning of the postembryonic mesoderm (Shen et al. 2018; Suzuki et al. 1999). Interestingly, although Chordin is lost in advanced nematodes, it was found in early branching nematodes Romanomermis culicivorax and Trichinella spiralis as well as in a member of a closely related ecdysozoan phylum Nematomorpha, Gordius sp. (Kraus 2015). However, the function of Chordin and BMP signalling in general in these animals remains unknown.
Deuterostomia
(8) Echinodermata
Although pentaradially symmetric as adults, echinoderm larvae are bilaterally symmetric and have a BMP-dependent DV axis, whose establishment and patterning have been analysed in sea urchin embryos. Unusually, the sea urchin gene with the earliest known bilaterally symmetric expression is Nodal, which is activated on the future ventral side of the Paracentrotus lividus blastula and activates the expression of bmp2/4 and admp1, but also chordin (Chang et al. 2016; Duboc et al. 2004; Lapraz et al. 2009, 2015). Whilst elegant experiments have shown that Chordin is required to shuttle BMP ligands and promote BMP signalling on the dorsal side, BMP itself (in the absence of Chordin) can phosphorylate Smad1/5 throughout the embryo, albeit only to a low level (Lapraz et al. 2009, 2015). ADMP2 is a dorsally-expressed ligand that additionally promotes dorsal pSmad1/5 and is under positive control of BMP signalling (Chang et al. 2016; Lapraz et al. 2015).
(9) Hemichordata
DV axis patterning was examined in two acorn worm species, the equally cleaving direct developer Saccoglossus kowalevskii (Lowe et al. 2006) and the unequally cleaving indirect developer Ptychodera flava (Su et al. 2019). In both cases, BMP signalling is required for secondary axis patterning. The BMP ligands (BMP2/4 and, in the case of Saccoglossus, also BMP5-8) are expressed dorsally, whereas Chordin is expressed on the ventral side (Lowe et al. 2006; Röttinger et al. 2015; Röttinger and Martindale 2011; Su et al. 2019). Treating embryos with recombinant zebrafish BMP4 leads to dorsalisation, whilst inhibiting BMP signalling pharmacologically or by BMP2/4 knockdown results in ventralisation (Lowe et al. 2006; Röttinger et al. 2015; Su et al. 2019). Also ADMPs are found in hemichordates: ADMP2 is expressed dorsally in Ptychodera (Chang et al. 2016; Röttinger et al. 2015), whereas ADMP1—the orthologue of the Saccoglossus ADMP (Lowe et al. 2006)—is under negative control of BMP signalling and expressed ventrally together with Chordin. Some fragments of the gene regulatory network responsible for the hemichordate DV patterning are clearly still unknown, especially the role of Nodal, which, like in the sea urchin, is expressed on the ventral side of the Ptychodera embryo. Surprisingly, treating the embryos with recombinant mouse Nodal resulted in the same dorsalising effect as the treatment with recombinant zebrafish BMP4 (Röttinger et al. 2015), suggesting the Nodal signalling might activate the expression of a ventrally expressed BMP (maybe it is ADMP in Saccoglossus) and its shuttling by Chordin.
(10) Chordata
In the early gastrula of the lancelet Branchiostoma, a member of the cephalochordates, BMP2/4 and BMP5-8 are expressed vegetally without DV asymmetry, whereas Chordin and ADMP are expressed dorsally. This situation changes at later gastrula stages when BMP2/4 and BMP5-8 expression becomes suppressed dorsally (Yu et al. 2007). Perturbation of BMP signalling has shown that its inhibition leads to dorsalisation and activation of BMP signalling leads to ventralisation (Le Petillon et al. 2017; Onai et al. 2010; Yu et al. 2007). Already in cephalochordates we see the persistent chordate theme of the opposing activities of the dorsalising Nodal and the ventralising BMP (Onai et al. 2010). BMP signalling is also important for the downstream left–right asymmetry (Soukup and Kozmik 2018).
Work on sea squirts, which is a common name for ascidians—the members of the vertebrate sister group Tunicata, showed that BMP signalling mediated by the dorso-laterally expressed ADMP, and the ventrally expressed BMP2/4 (and possibly also BMP5-8) is active in the ventral epidermis from late gastrula stages on (Imai et al. 2012; Roure et al. 2023; Waki et al. 2015). Chordin is expressed in two dorso-lateral stripes (Abitua et al. 2015) suggesting that it might be involved in antagonizing BMP activity on the dorsal side of the embryo. BMP signalling is required for the ectodermal DV patterning (Imai et al. 2012; Roure et al. 2023; Waki et al. 2015). ADMP-mediated BMP signalling and Chordin are also involved in the formation of the three palps on the head of the tadpole, which are specified in the pSmad1/5-negative domain at the so-called anterior neural border but subsequently require BMP signalling for their proper patterning (Darras and Nishida 2001; Roure et al. 2023; Liu et al. 2023). It has been suggested that ascidian BMP signalling has an “anti-neural” function during neural induction, since pharmacological inhibition of BMP signalling de-represses the dorsally expressed pro-neural transcription factor Otx (Ohta and Satou 2013). However, this statement, in our opinion, has to be amended. Similar to the situation in cephalochordates, BMP signalling in tunicates cannot be considered strictly anti-neural, as it is required for the specification of the ventral sensory neurons in both these lineages (Waki et al. 2015; Lu et al. 2012).
Finally, we come to the situation in vertebrates. Over the last 30 years, the zebrafish Danio rerio has become a powerful model system to investigate the functions of BMP signalling during vertebrate development. BMP signalling activity has been shown by pSmad1/5 immunofluorescence to be restricted to the ventral side during early gastrulation (Greenfeld et al. 2021; Pomreinke et al. 2017; Ramel and Hill 2013; Rogers et al. 2020; Tucker et al. 2008; Zinski et al. 2017). The expression of BMP ligands BMP4 and BMP2b is restricted to ventral tissues and, curiously, the dorsal organizer, during early gastrulation (Dick et al. 2000; Kishimoto et al. 1997; Ramel and Hill 2013; Schmid et al. 2000; Xue et al. 2014). Mutants for the BMP ligands BMP2b and BMP7a lack ventral tissues, such as the tail and blood (Dick et al. 2000; Mullins et al. 1996; Schmid et al. 2000). Chordin is expressed dorsally in the organizer (Hammerschmidt et al. 1996; Kishimoto et al. 1997; Miller-Bertoglio et al. 1997). Loss of Chordin function leads to dorsal expansion of the pSmad1/5 domain and dorsal expansion of ventral marker genes (Hammerschmidt et al. 1996; Pomreinke et al. 2017; Zinski et al. 2017). The mechanisms underlying BMP signalling-mediated patterning have been intensively studied over the last years. Amongst the tested models was also the shuttling of BMP ligands by Chordin, which we discussed above. In zebrafish, however, Chordin does not exhibit any pro-BMP functions and merely seems to dampen BMP signalling, as evident from chordin mutants that have an expanded, but not flattened pSmad1/5 gradient (Pomreinke et al. 2017; Zinski et al. 2017). Moreover, extracellular dispersion of Chordin, which is crucial for Chordin function in the shuttling model (Ashe and Levine 1999), is not required for its function in zebrafish, as shown by experiments using a membrane-tethered Chordin (Tuazon et al. 2020). We have to note that the dorsally-expressed ADMP also functions in dorsoventral patterning (Dickmeis et al. 2001; Lele et al. 2001; Yan et al. 2019), and it can currently not be excluded that Chordin shuttles this BMP ligand as in frogs (Pomreinke et al. 2017; Reversade and De Robertis 2005; Reversade et al. 2005). BMPs are usually considered textbook examples of morphogens acting to pattern the DV axis (Bier and De Robertis 2015). However, the quantification of the pSmad1/5 gradient and BMP target gene expression in zebrafish has revealed that the classical threshold-dependent target gene activation is true only to a certain extent, and that crosstalk between signalling pathways is critical to pattern complex tissues (Greenfeld et al. 2021; Rogers et al. 2020).
Analysis of the DV patterning in amphibians started somewhat gloriously with the discovery of the dorsalizing, axis-inducing activity conveyed by the Spemann organizer (Spemann and Mangold 1924). Chordin was first identified as an organizer gene with the potency to induce axis duplication upon misexpression in the frog Xenopus (Sasai et al. 1994). Chordin binds the ventrally-expressed BMP4 as well as BMP4-BMP7 heterodimers, and blocks the BMP ligand from binding to its receptors (Dale et al. 1992; Piccolo et al. 1996). BMP4 itself ventralises embryos and represses dorsal organizer genes like goosecoid (Fainsod et al. 1994; Jones et al. 1996). The proper formation of ventral structures requires in addition to BMP4 the dorsally-produced ADMP, as well as BMP2 and BMP7, which are expressed along the whole DV axis (Hawley et al. 1995; Hemmati-Brivanlou and Thomsen 1995). Only a combined loss of BMP2, BMP4, BMP7 and ADMP leads to a radialisation of the frog embryo (Reversade and De Robertis 2005). Although the general components of dorsoventral patterning and their expression domains are very similar to those in zebrafish embryos, a more active function of Chordin in the patterning of Xenopus was proposed. There is evidence that Chordin does not only act as a dorsal inhibitor of BMP signalling, but also helps in the shuttling of BMPs to the ventral side via the ECM of the Brachet’s cleft (Ben-Zvi et al. 2008; Plouhinec et al. 2013).
Studying mammalian embryogenesis is difficult due to the intrauterine development and thus lacking accessibility of the embryo. Therefore, little functional data on early mammalian dorsoventral patterning is available. Multiple BMP ligands are expressed during and are crucial for early mouse development, albeit partially redundant (Lyons et al. 1995; Solloway and Robertson 1999; Winnier et al. 1995). Chordin is expressed in the mouse organizer, the node, but is not required for the gross patterning of embryonic axes due to partial redundancy with the BMP inhibitor Noggin (Bachiller et al. 2000). Early in mouse development, BMP4 is expressed exclusively in extraembryonic tissue (Bachiller et al. 2000; Beck et al. 2002; Ben-Haim et al. 2006), and BMP4 signalling to the embryonic cells is important for primitive streak formation (reviewed in (Arnold and Robertson 2009)). With the embryo largely inaccessible, certain aspects of cellular communication and tissue patterning can be reconstructed in vitro using cell culture, gastruloids and blastoids. A synthetic system of BMP/Chordin secreting cells was shown to pattern a 2D culture, in agreement with BMP shuttling (Zhu et al. 2023). Furthermore, an increasing number of model systems derived from mouse or human embryonic stem cells (mESCs/hESCs) provide fascinating, developmentally-relevant insights. It was shown that colonies of hESCs can be patterned using BMP4 that is provided via the medium (Etoc et al. 2016). The resulting radial patterning within the colonies is the result of a reaction–diffusion system that requires Noggin. Especially gastruloids, 3D assemblies of ESCs that can partially recapitulate tissue differentiation and morphogenesis during development, will be useful systems to investigate mammalian DV patterning in the future. Mouse gastruloids, for example, break the initial symmetry and self-organize to form anterior–posterior as well as dorsoventral axes, without input from extraembryonic tissues (Beccari et al. 2018). The blastocyst-like blastoids, on the other hand, provide a valuable model system to study the communication between embryonic cells and extraembryonic trophectoderm (Rivron et al. 2018). In this context, it was shown that embryonically produced BMP4 signals to the trophectoderm, where it regulates proliferation and morphogenesis together with Nodal.
In summary, the central role of BMP2/4 and Chordin in the patterning of the DV axis is clearly ancestral for Bilateria. Mapping the orientation of the BMP signalling gradients in different bilaterian models on the phylogenetic tree tells us that the last common ancestor of Bilateria had to have a dorsal-to-ventral BMP signalling gradient—like in protostomes (Spiralia + Ecdysozoa) and non-chordate deuterostomes. A ventral BMP signalling maximum characteristic for cephalochordates and vertebrates suggests an axis flip at the base of Chordata, which made us, rather than the famous Geoffroy Saint-Hilaire’s lobster,Footnote 4 “inverted” (Arendt and Nübler-Jung 1994; Geoffroy Saint-Hilaire 1822; Su et al. 2019). As bilaterians diversified, the BMP/Chordin-based patterning system underwent independent modifications sometimes involving the loss of Chordin. Moreover, in some clades, BMP signalling was “decommissioned” from DV patterning altogether and replaced by other mechanisms. Having established that, let us now address the situation with the BMP signalling outside Bilateria.
BMP signalling in non-Bilateria
To-date, we know of four non-bilaterian phyla: Ctenophora (comb jellies), Porifera (sponges), Placozoa, and Cnidaria (corals and jellyfish). Whilst the phylogenetic positions of Ctenophora and Porifera have been a matter of intense debate during the last 15 years, all recent phylogenies retrieve Cnidaria as a robust bilaterian sister group, with Placozoa placed as a sister group to Bilateria + Cnidaria (Dunn et al. 2008; Feuda et al. 2017; Moroz et al. 2014; Pisani et al. 2015; Ryan et al. 2013; Schultz et al. 2023; Simion et al. 2017; Whelan et al. 2015) (Fig. 2). Ctenophores demonstrate a curious type of body symmetry: they are biradial. This means that they have a single oral-aboral (OA) body axis, and two axes of symmetry—one along their slit-like mouth, and another one perpendicular to it passing through the bases of their tentacles. Phylogenetic analyses of the available ctenophore sequences suggest that ctenophores do not seem to have Chordin and BMP orthologues, although several proteins clearly belong to the TGFβ superfamily of ligands (Genikhovich and Technau 2017; Pang et al. 2011).
Adult sponges are sessile and mostly lack a clear body axis (with the exception of asconoid and syconoid calcareous sponges, which are very clearly radially symmetric as adults), however, during their embryonic stages, all sponges display radial symmetry. The situation in sponges is intriguing: the genome of a homoscleromorph sponge Oscarella (Nichols et al. 2012) harbours a clear Chordin orthologue, however there is no statistical support for its several TGFβ molecules being bona fide BMPs. Other sequenced sponges do not seem to have either BMPs or Chordin (Kenny et al. 2020; Santini et al. 2023; Leininger et al. 2014).
Placozoans have been traditionally viewed as animals without any obvious symmetry and with the “dorsal” surface facing the water and “ventral” surface facing the substrate on which they crawl. Analyses by DuBuc and colleagues strongly suggest, however, that their “ventral” surface should be considered the oral, and their “dorsal” surface—the aboral end of their OA axis along which they became flattened (DuBuc et al. 2019). There is no trace of the second body axis either at the morphological or the molecular level. Trichoplax has a chordin-like molecule (DuBuc et al. 2019) grouping with vertebrate Kielin proteins, but no “real” Chordin, as well as several BMP molecules grouping with cnidarian and bilaterian BMPs albeit with low support.
Cnidaria (Fig. 3A), the bilaterian sister group, are divided into two clades: Medusozoa and Anthozoa (McFadden et al. 2021; Zapata et al. 2015). Medusozoa encompass the “conventional” cnidarian classes Hydrozoa, Staurozoa, Scyphozoa and Cubozoa, which we will address in some detail, as well as the parasitic Endocnidozoa, which were only recently recognized as cnidarians and will not be considered further in this review (Kayal et al. 2018). Anthozoa consist of Octocorallia (soft corals, sea pens etc.) and Hexacorallia (hard corals, sea anemones etc.) and are the slower evolving clade retaining many genes lost in medusozoans (Chapman et al. 2010; Gold et al. 2019; Hu et al. 2020; Khalturin et al. 2019; Leclère et al. 2019; McFadden et al. 2021; Putnam et al. 2007; Shinzato et al. 2011). Traditionally, cnidarians are described as radially symmetric, which is true for Medusozoa, but not for Anthozoa. The bilaterality of the anthozoan anatomy, which in some cases is secondarily replaced by biradiality, has been an accepted fact since at least the beginning of the twentieth century (Pax 1925; Stephenson 1928). In addition to the Wnt-dependent OA axis, which is a shared feature of all cnidarians, anthozoans have a second, “directive” axis perpendicular to the OA axis. At the level of morphology, bilateral symmetry manifests itself in the order of the formation of the gastrodermal folds called mesenteries, which subdivide the gastric cavity of anthozoans into mesenterial chambers sometimes referred to as segments, the position of the muscles on these mesenteries, and in the slit-like shape of the pharynx, which usually has a ciliated groove—the siphonoglyph—on one of its ends (Fig. 3B, C) (Berking 2007; Pax 1925; Stephenson 1928). All this did not prevent various textbooks from using sea anemones as examples of an animal with radial symmetry—they looked too much like a flower.

Modified from Kükenthal (1925). C Cross-section of the hexacoral Halcampa duodecim-cirrata (a twelve-tentacle burrowing sea anemone). Modified from Pax 1925. On B and C, orange lines show the orientation of the directive axis; arrowheads show the bilateral orientation of the retractor muscles on the mesenteries
Cnidarian BMP signalling components and cnidarian bilaterality. A Cnidarian phylogeny and distribution of the key BMP-related traits in cnidarian classes. B Cross-section of the octocoral Alcyonium digitatum (dead man’s fingers).
All intracellular components of BMP signalling (type I and type II BMP receptors, SMAD1/5, SMAD4) are present throughout Cnidaria, however, the situation is different when it comes to the secreted components of the BMP signalling, especially to the “core” BMP ligands BMP2/4 and BMP5/8, and their antagonists (Fig. 3A). Both, Chordin and BMP2/4 orthologues are missing from the genomes of the staurozoans and cubozoans sequenced to-date (Kayal et al. 2018; Khalturin et al. 2019); the hydrozoans Clytia, Hydractinia and Hydra have lost Chordin, and have a TGFβ molecule, which may be a derived BMP2/4, whilst the scyphozoan Aurelia has a clear Chordin orthologue but no apparent BMP2/4 has been published so far (Genikhovich and Technau 2017). Curiously, all medusozoans, which have been looked at, possess a BMP5-8 orthologue, sometimes in several copies (Genikhovich and Technau 2017). BMP5-8 and BMP2/4 are expressed in specific radially symmetric domains along the oral-aboral (OA) axis in Hydra (Reinhardt et al. 2004; Watanabe et al. 2014), and there is an interesting report of a bilaterally symmetric expression of the putative BMP2/4 and BMP5-8 at the aboral pole of the early planula larva in the hydroid Podocoryne (= Hydractinia) carnea (Reber-Müller et al. 2006); however, this result, in our opinion, is so unexpected and, potentially, so important that it needs to be independently confirmed.
In contrast to the medusozoans, anthozoans lost much fewer genes in the course of evolution (Fig. 3A). In the hexacorallian genomes we always find orthologues of BMP2/4 and Chordin (as well as BMP5-8, GDF5, ADMP, Gremlin, Noggin, Follistatin etc.) (Putnam et al. 2007; Shinzato et al. 2011), however, the octocoral Xenia appears to lack a Chordin orthologue (Hu et al. 2020). Curiously, the most experimentally developed hexacoral model—the sea anemone Nematostella vectensis—lost one BMP inhibitor, Cerberus, which was retained in the hydrozoan Clytia. The wider appreciation of anthozoan bilaterality came after the publication of the expression pattern of BMP2/4 on one side of the blastopore in a gastrula of the stony coral Acropora millepora soon followed by a similar observation in the sea anemone Nematostella: anthozoan embryos were clearly bilateral at the molecular level long before the first signs of morphological bilaterality became evident (Finnerty et al. 2004; Hayward et al. 2002; Matus et al. 2006a, b; Rentzsch et al. 2006).
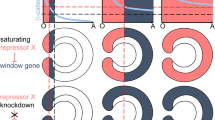
Thanks to the availability of the gene knockdown techniques we now know that, in Nematostella, the directive axis forms due to a BMP signalling-dependent symmetry break in the initially radially symmetric expression of the core BMP ligands BMP2/4 and BMP5/8, as well as of the BMP antagonist Chordin. This symmetry break establishes a gradient of BMP signalling along the newly formed directive axis, and the knockdown of any of these components leads to the loss of the gradient and complete radialisation of the embryo at the molecular and morphological level. Other components, such as the BMP ligand GDF5-like, the secreted BMP antagonist Gremlin, the putative BMP co-receptor RGM, the nuclear modulator of the pSMAD1/5 and potential transcriptional co-repressor ZSWIM4-6, and most certainly some other yet uncharacterized players, help shape the BMP signalling gradient and facilitate its robustness (Genikhovich et al. 2015; Knabl et al. 2024; Leclère and Rentzsch 2014; Saina et al. 2009). There are two notable features of the Nematostella BMP-dependent axis establishment and patterning: (i) BMP2/4 and BMP5-8 expression is negatively regulated by BMP signalling (Saina et al. 2009; Genikhovich et al. 2015; Knabl et al. 2024); (ii) at early stages, BMP signalling is highly dependent on Chordin. In Nematostella, Chordin is expressed on the same side of the directive axis as BMP2/4 and BMP5/8, although their expression domains about each other (Fig. 4A). Antibody staining against nuclear pSMAD1/5 shows that the BMP signalling gradient forms at late gastrula stage and has its maximum on the Chordin/BMP2/4/BMP5-8-negative side of the directive axis, where BMP signalling activates the transcription of GDF5-like (another BMP) and of the BMP antagonist Gremlin, which help shape and maintain the signalling gradient (Fig. 4A). In late planula larva, Chordin expression stops, whilst BMP expression continues, however, the gradient of nuclear pSMAD1/5 along the directive axis disappears concomitantly with the shutdown of Chordin expression, and nuclear pSMAD1/5 starts to be observed in the same domain where the BMP2/4 and BMP5-8 genes are expressed (Knabl et al. 2024). Chordin and BMP2/4 co-expression is very unusual but not unheard of in Bilateria. Similar to the situation in Nematostella, Chordin and BMP2/4 are co-expressed on the ventral side of the embryo of the sea urchin Paracentrotus lividus, and ventrally produced Chordin is required for the establishment of the dorsal nuclear pSMAD1/5 maximum, which is suggestive of the Chordin-dependent BMP shuttling, just like in Drosophila or frog. However, like in other Bilateria, sea urchin Chordin knockdown results in the expansion of the BMP signalling domain (Lapraz et al. 2009, 2015), not so in the sea anemone. Nematostella Chordin knockdown results not in the upregulation of the BMP signalling but in its severe reduction and in the loss of the BMP signalling gradient in late gastrula/early planula (Genikhovich et al. 2015). Thus, it appears that Nematostella Chordin is not only responsible for the establishment of the pSMAD1/5 gradient with a maximum opposite to the Chordin source, but BMP signalling at the early stage is in principle Chordin-dependent. The current model is that Nematostella Chordin represses local BMP2/4/BMP5-8-mediated signalling thus preventing transcriptional repression of BMP2/4 and BMP5-8. At the same time, Chordin shuttles BMP2/4/BMP5-8 heterodimers promoting BMP signalling (reinforced by the locally produced GDF5-like) at the opposite side of the directive axis. In the future, it will be interesting to understand the molecular mechanism of this apparent dependence of BMP signalling on Chordin at one developmental stage but not at another.
In Nematostella, BMP signalling controls directive axis patterning by regulating Hox genes. A Expression domains of the BMP ligands and BMP inhibitors in Nematostella early planula. B Expression of Gbx and Hox genes activated by BMP signalling in specific staggered domains along the directive axis of the Nematostella early planula and formation of the mesenteries at the boundaries of the Gbx/Hox expression. C In the primary polyp, each mesenterial segment acquires a unique Gbx/Hox code (shown on the left side of the cross-section). However, the fate of the segment is defined by one specific Hox gene or Gbx (shown on the right side of the cross-section) in a “posterior prevalence”-like manner. Knockouts of Gbx and Hox genes lead to specific homeotic transformations, where the mesenterial boundary disappears; the segment normally specified by the mutated Gbx/Hox gene fuses with and acquires the fate of its neighbouring segment located towards the BMP2/4 side of the directive axis. Please note that paired segments are called S2, S3, and S4 here rather than S2/S8, S3/S7, and S4/S6 as in He et al. (2018). D Staggered Hox expression of the bilaterian Hox genes, which demonstrate a similar regulatory behaviour. Animal sketches on D were reproduced with permission from Technau and Genikhovich 2018
Taken together, we have two sister clades—Bilateria and Cnidaria—in which we find bilaterally symmetric animals with two orthogonal body axes patterned by graded Wnt (posterior-anterior and OA, respectively) and BMP signalling (DV and directive, respectively). Thus, it is possible that the last common ancestor of Cnidaria and Bilateria may have been bilaterally symmetric, that cnidarian and bilaterian body axes may be homologous, and that radially symmetric medusozoans may have lost their second body axis. Given the recurrent loss of BMP2/4 or Chordin or both in different medusozoans it is tempting to speculate that this may be something more than a mere correlation. Should a radially symmetric medusozoan with a full set of “core BMPs” and Chordin be found, it would be interesting to explore whether or not these medusozoan BMPs and Chordin would be able to functionally substitute for their Nematostella counterparts, for which we know that they can generate bilaterality. However, there is a catch: we cannot rule out the possibility of convergent evolution of the BMP-dependent second axis in Bilateria and Anthozoa simply because BMP and Chordin represent a “symmetry breaking machine”. In one of his last papers, Hans Meinhardt analysed what was known about the regulatory relationships between Wnt and BMP in Cnidaria at the time and suggested that once the regulatory architecture was correct, it was “easy” to spontaneously create and maintain bilaterality (Meinhardt 2015). There is at least some experimental evidence in favour of this arguably even more exciting scenario.
Potential convergence
We and others have shown that different intensities of Wnt/β-catenin signalling result in the activation of specific sets of genes in the oral “high Wnt” and in the aboral “low Wnt” areas of the Nematostella gastrula (Kraus et al. 2016; Lebedeva et al. 2021; Marlow et al. 2013; Röttinger et al. 2012). These sets of genes—Brachyury, FoxA, FoxB, and Six3/6, FoxQ2, Fz5/8—are canonical bilaterian posterior and anterior markers, respectively. Moreover, the regulatory logic of this patterning in Nematostella is like in deuterostomes; hence, we suggested that cnidarian OA axis is homologous to the bilaterian posterior-anterior axis (Lebedeva et al. 2021). The situation with the BMP-dependent axis is different. Part of global DV patterning in bilaterians with centralized nervous systems is the patterning of the neuroectoderm. In a classical example, BMP similarly affects neurectoderm patterning in Drosophila and Xenopus: in the dorsalmost area with the highest levels of BMP signalling, Msh is activated (Msx1 in Xenopus; expressed in the ventralmost cells of the neural plate); in the intermediate levels of BMP signalling, ind is expressed (Gsh2 in Xenopus); in the ventralmost neurectoderm with the lowest levels of BMP signalling, vnd is expressed (Nkx6.1 in Xenopus; expressed in the cells of the dorsal midline) (Bier and De Robertis 2015; Fig. 5). Once activated in their respective domains, these transcription factors form a regulatory network based on mutual repressive interactions, which patterns the neurectoderm (Sagner and Briscoe 2019; Weiss et al. 1998). The orthologues of these genes exist in Nematostella (Zimmermann et al. 2023) but they do not seem to be controlled by BMP. Instead, graded BMP signalling is regulating axial patterning by directly activating a set of Hox genes and a non-Hox Antennapedia class homeobox gene Gbx. (Genikhovich et al. 2015; Knabl et al. 2024) (Fig. 4B). He et al. showed that their staggered expression is responsible for the formation of the mesenteries and providing each of the three paired (S2, S3, and S4) and two unpaired mesenterial chambers (S1 and S5) with unique molecular identities (He et al. 2018; Ryan et al. 2007; Fig. 4B, C). In this process, Nematostella Hox genes and Gbx display something suspiciously similar to the “posterior prevalence”, as we know it from vertebrates and arthropods. HoxE/Anthox1a, which is expressed in the unpaired mesenterial chamber (= segment) S5 at the former “high BMP signalling” end of the directive axis functionally overrides HoxD/Anthox8 expressed in the segment pair S4 and S5. HoxD/Anthox8 functionally overrides HoxB/Anthox6a expressed in the segments S3, S4, and S5. Finally, HoxB/Anthox6a functionally overrides Gbx expressed in the segments S2, S3, S4, and S5 (Fig. 4C). The mechanism of this “prevalence” is unknown and needs to be experimentally addressed. The obvious difference to the bilaterian Hox genes is that, in Nematostella, Hox genes are involved not only in conveying axial identities to segments, but in the formation of the segment boundaries. Staggered expression of the Nematostella Hox genes along the directive axis was so suggestive that it revived the nineteenth century idea that anthozoan directive axis may be homologous to the bilaterian anterior–posterior axis (Fig. 4C, D) (Arendt et al. 2016; Jägersten 1955; Nielsen et al. 2018; Remane 1950; Sedgwick 1884; van Beneden 1891). This remains a matter of debate, given that cnidarian OA axis shares many regulatory features with the bilaterian posterior-anterior axis, that bilaterian Hox genes are expressed along the posterior-anterior body axis, which is controlled by Wnt rather than by BMP, and that cnidarian Hox genes are most likely not direct orthologues of the bilaterian Hox genes but rather a product of the Cnidaria-specific diversification of the Hox cluster of the cnidarian-bilaterian ancestor, which likely contained a single “anterior” and a single “non-anterior” Hox gene (reviewed in more detail in Genikhovich and Technau 2017). Possibly, however, these ancestral Hox genes were already expressed in staggered domains and capable of overriding each other’s function.

Reproduced with permission from Bier and De Robertis (2015)
BMP signalling gradient regulates orthologous genes during neurectoderm patterning in the fly and in the frog.
Conclusion
With this review, we cannot answer the question whether the last common ancestor of Cnidaria and Bilateria was bilaterally or radially symmetric or whether the emergence of the Chordin-mediated BMP shuttling was the evolutionary event, which made bilaterally symmetric body plans possible. However, we hope that we managed to convey a different message: BMP signalling-dependent patterning of the secondary body axis in animals is at the same time very conserved and very evolvable. Often, superficial differences originate from a detectable ancestral pattern, whilst superficial similarities may arise convergently based on the intrinsic properties of the BMP-Chordin system to break symmetry and activate downstream transcription factors in a concentration-dependent manner.
Data availability
No datasets were generated or analysed during the current study.
Notes
Although vertebrates also have Smad8 and Smad9, we will to refer to all the Smad1/5/8/9 homologs as Smad1/5 in this article.
In this review, we will mention Drosophila gene and protein names when we encounter them for the first time, but we will then keep using their vertebrate orthologue names even when talking about Drosophila genes and proteins to keep the terminology consistent.
Phylogenetic analyses showed that xenacoelomorphs Xenoturbella bocki and Symsagittifera roscoffensis have two ADMP genes, which are orthologous to the ADMP1 and ADMP2 of ambulacrarians (Chang et al. 2016). This can be seen either as additional evidence in favour of the phylogenetic position of Xenacoelomorpha as a sister to Ambulacraria or indicate multiple independent losses of ADMP2 across Bilateria.
Étienne Geoffroy Saint-Hilaire (1772–1844)—a French naturalist who noticed that the relative positions of the central nervous system, heart and gut in a lobster were the same as in a mammal but the ventral most lobster structure corresponded to the dorsal most mammalian structure, hence the name for the “inverted lobster hypothesis”.
References
Abitua PB, Gainous TB, Kaczmarczyk AN, Winchell CJ, Hudson C, Kamata K, Nakagawa M, Tsuda M, Kusakabe TG, Levine M (2015) The pre-vertebrate origins of neurogenic placodes. Nature 524:462–465
Akiyama-Oda Y, Oda H (2006) Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development 133:2347–2357
Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y (1995) Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochem Biophys Res Commun 210:670–677
Arendt D, Nübler-Jung K (1994) Inversion of dorsoventral axis? Nature 371:26
Arendt D, Tosches MA, Marlow H (2016) From nerve net to nerve ring, nerve cord and brain–evolution of the nervous system. Nat Rev Neurosci 17:61–72
Arnold SJ, Robertson EJ (2009) Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol 10:91–103
Arora K, Levine MS, O’Connor MB (1994) The screw gene encodes a ubiquitously expressed member of the TGF-β family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev 8:2588–2601
Ashe HL, Levine M (1999) Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature 398:427–431
Babonis LS, Martindale MQ (2017) Phylogenetic evidence for the modular evolution of metazoan signalling pathways. Philos Trans R Soc Lond B Biol Sci 372:1713
Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM (2000) The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403:658–661
Bauer M, Aguilar G, Wharton KA, Matsuda S, Affolter M (2023) Heterodimerization-dependent secretion of bone morphogenetic proteins in Drosophila. Dev Cell 58(645–659):e644
Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy AC, Lutolf MP, Duboule D, Arias AM (2018) Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562:272–276
Beck S, Le Good JA, Guzman M, Ben Haim N, Roy K, Beermann F, Constam DB (2002) Extraembryonic proteases regulate Nodal signalling during gastrulation. Nat Cell Biol 4:981–985
Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB (2006) The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell 11:313–323
Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N (2008) Scaling of the BMP activation gradient in Xenopus embryos. Nature 453:1205–1211
Berking S (2007) Generation of bilateral symmetry in Anthozoa: a model. J Theor Biol 246:477–490
Bier E, De Robertis EM (2015) BMP gradients: a paradigm for morphogen-mediated developmental patterning. Science 348:aaa5838
Brazil DP, Church RH, Surae S, Godson C, Martin F (2015) BMP signalling: agony and antagony in the family. Trends Cell Biol 25:249–264
Cannon JT, Vellutini BC, Smith J 3rd, Ronquist F, Jondelius U, Hejnol A (2016) Xenacoelomorpha is the sister group to Nephrozoa. Nature 530:89–93
Chang C, Holtzman DA, Chau S, Chickering T, Woolf EA, Holmgren LM, Bodorova J, Gearing DP, Holmes WE, Brivanlou AH (2001) Twisted gastrulation can function as a BMP antagonist. Nature 410:483–487
Chang YC, Pai CY, Chen YC, Ting HC, Martinez P, Telford MJ, Yu JK, Su YH (2016) Regulatory circuit rewiring and functional divergence of the duplicate admp genes in dorsoventral axial patterning. Dev Biol 410:108–118
Chapman JA, Kirkness EF, Simakov O, Hampson SE, Mitros T, Weinmaier T, Rattei T, Balasubramanian PG, Borman J, Busam D, Disbennett K, Pfannkoch C, Sumin N, Sutton GG, Viswanathan LD, Walenz B, Goodstein DM, Hellsten U, Kawashima T, Prochnik SE, Putnam NH, Shu S, Blumberg B, Dana CE, Gee L, Kibler DF, Law L, Lindgens D, Martinez DE, Peng J, Wigge PA, Bertulat B, Guder C, Nakamura Y, Ozbek S, Watanabe H, Khalturin K, Hemmrich G, Franke A, Augustin R, Fraune S, Hayakawa E, Hayakawa S, Hirose M, Hwang JS, Ikeo K, Nishimiya-Fujisawa C, Ogura A, Takahashi T, Steinmetz PR, Zhang X, Aufschnaiter R, Eder MK, Gorny AK, Salvenmoser W, Heimberg AM, Wheeler BM, Peterson KJ, Bottger A, Tischler P, Wolf A, Gojobori T, Remington KA, Strausberg RL, Venter JC, Technau U, Hobmayer B, Bosch TC, Holstein TW, Fujisawa T, Bode HR, David CN, Rokhsar DS, Steele RE (2010) The dynamic genome of Hydra. Nature 464:592–596
Clark EG, Petersen CP (2023) BMP suppresses WNT to integrate patterning of orthogonal body axes in adult planarians. PLoS Genet 19:e1010608
Cui Y, Hackenmiller R, Berg L, Jean F, Nakayama T, Thomas G, Christian JL (2001) The activity and signaling range of mature BMP-4 is regulated by sequential cleavage at two sites within the prodomain of the precursor. Genes Dev 15:2797–2802
Dale L, Howes G, Price BM, Smith JC (1992) Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development 115:573–585
Dal-Pra S, Furthauer M, Van-Celst J, Thisse B, Thisse C (2006) Noggin1 and Follistatin-like2 function redundantly to Chordin to antagonize BMP activity. Dev Biol 298:514–526
Darras S, Nishida H (2001) The BMP/CHORDIN antagonism controls sensory pigment cell specification and differentiation in the ascidian embryo. Dev Biol 236:271–288
Denes AS, Jekely G, Steinmetz PR, Raible F, Snyman H, Prud’homme B, Ferrier DE, Balavoine G, Arendt D (2007) Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 129:277–288
Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, Talbot WS, Bouwmeester T, Hammerschmidt M (2000) Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development 127:343–354
Dickmeis T, Rastegar S, Aanstad P, Clark M, Fischer N, Korzh V, Strahle U (2001) Expression of the anti-dorsalizing morphogenetic protein gene in the zebrafish embryo. Dev Genes Evol 211:568–572
Duboc V, Rottinger E, Besnardeau L, Lepage T (2004) Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev Cell 6:397–410
DuBuc TQ, Ryan JF, Martindale MQ (2019) “Dorsal-ventral” genes are part of an ancient axial patterning system: evidence from Trichoplax adhaerens (Placozoa). Mol Biol Evol 36:966–973
Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sorensen MV, Haddock SH, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G (2008) Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452:745–749
Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N (2002) Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature 419:304–308
Etoc F, Metzger J, Ruzo A, Kirst C, Yoney A, Ozair MZ, Brivanlou AH, Siggia ED (2016) A balance between secreted inhibitors and edge sensing controls gastruloid self-organization. Dev Cell 39:302–315
Fainsod A, Steinbeisser H, De Robertis EM (1994) On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J 13:5015–5025
Ferguson EL, Anderson KV (1992a) Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell 71:451–461
Ferguson EL, Anderson KV (1992b) Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development 114:583–597
Feuda R, Dohrmann M, Pett W, Philippe H, Rota-Stabelli O, Lartillot N, Wörheide G, Pisani D (2017) Improved modeling of compositional heterogeneity supports sponges as sister to all other animals. Curr Biol 27:e3864
Finnerty JR, Pang K, Burton P, Paulson D, Martindale MQ (2004) Origins of bilateral symmetry: Hox and dpp expression in a sea anemone. Science 304:1335–1337
Francois V, Solloway M, O’Neill JW, Emery J, Bier E (1994) Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev 8:2602–2616
Fritsch C, Sawala A, Harris R, Maartens A, Sutcliffe C, Ashe HL, Ray RP (2012) Different requirements for proteolytic processing of bone morphogenetic protein 5/6/7/8 ligands in Drosophila melanogaster. J Biol Chem 287:5942–5953
Gavino MA, Reddien PW (2011) A Bmp/Admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. Curr Biol 21:294–299
Gavin-Smyth J, Wang YC, Butler I, Ferguson EL (2013) A genetic network conferring canalization to a bistable patterning system in Drosophila. Curr Biol 23:2296–2302
Genikhovich G, Technau U (2017) On the evolution of bilaterality. Development 144:3392–3404
Genikhovich G, Fried P, Prünster MM, Schinko JB, Gilles AF, Fredman D, Meier K, Iber D, Technau U (2015) Axis patterning by BMPs: cnidarian network reveals evolutionary constraints. Cell Rep 10:1646–1654
Geoffroy Saint-Hilaire E (1822) Considérations générales sur la vertèbre. Mém Mus D’hist Nat 9:89–112
Gold DA, Katsuki T, Li Y, Yan X, Regulski M, Ibberson D, Holstein T, Steele RE, Jacobs DK, Greenspan RJ (2019) The genome of the jellyfish Aurelia and the evolution of animal complexity. Nat Ecol Evol 3:96–104
Greenfeld H, Lin J, Mullins MC (2021) The BMP signaling gradient is interpreted through concentration thresholds in dorsal-ventral axial patterning. PLoS Biol 19:e3001059
Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nusslein-Volhard C (1996) dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123:95–102
Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW (1995) Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev 9:2923–2935
Hayward DC, Samuel G, Pontynen PC, Catmull J, Saint R, Miller DJ, Ball EE (2002) Localized expression of a dpp/BMP2/4 ortholog in a coral embryo. Proc Natl Acad Sci U S A 99:8106–8111
Hazama M, Aono A, Ueno N, Fujisawa Y (1995) Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem Biophys Res Commun 209:859–866
He S, Del Viso F, Chen CY, Ikmi A, Kroesen AE, Gibson MC (2018) An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis. Science 361:1377–1380
Hejnol A, Martindale MQ (2008) Acoel development indicates the independent evolution of the bilaterian mouth and anus. Nature 456:382–386
Heldin CH, Moustakas A (2016) Signaling receptors for TGF-beta family members. Cold Spring Harb Perspect Biol 8:a022053
Hemmati-Brivanlou A, Thomsen GH (1995) Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4. Dev Genet 17:78–89
Hill CS (2016) Transcriptional Control by the SMADs. Cold Spring Harb Perspect Biol 8:a022079
Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O’Connor MB, De Robertis EM, Ferguson EL (1996) The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell 86:607–617
Hoppe C, Bowles JR, Minchington TG, Sutcliffe C, Upadhyai P, Rattray M, Ashe HL (2020) Modulation of the promoter activation rate dictates the transcriptional response to graded BMP signaling levels in the Drosophila embryo. Dev Cell 54(727–741):e727
Hu M, Zheng X, Fan CM, Zheng Y (2020) Lineage dynamics of the endosymbiotic cell type in the soft coral Xenia. Nature 582:534–538
Huminiecki L, Goldovsky L, Freilich S, Moustakas A, Ouzounis C, Heldin CH (2009) Emergence, development and diversification of the TGF-beta signalling pathway within the animal kingdom. BMC Evol Biol 9:28
Iemura S, Yamamoto TS, Takagi C, Uchiyama H, Natsume T, Shimasaki S, Sugino H, Ueno N (1998) Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci U S A 95:9337–9342
Imai KS, Daido Y, Kusakabe TG, Satou Y (2012) Cis-acting transcriptional repression establishes a sharp boundary in chordate embryos. Science 337:964–967
Inomata H, Haraguchi T, Sasai Y (2008) Robust stability of the embryonic axial pattern requires a secreted scaffold for chordin degradation. Cell 134:854–865
Itoh K, Ossipova O, Sokol SY (2021) Pinhead antagonizes Admp to promote notochord formation. iScience 24:102520
Jägersten G (1955) On the early phylogeny of the Metazoa. Zool Bidr Uppsala 30:321–354
Jazwinska A, Rushlow C, Roth S (1999) The role of brinker in mediating the graded response to Dpp in early Drosophila embryos. Development 126:3323–3334
Jones CM, Dale L, Hogan BL, Wright CV, Smith JC (1996) Bone morphogenetic protein-4 (BMP-4) acts during gastrula stages to cause ventralization of Xenopus embryos. Development 122:1545–1554
Kapli P, Natsidis P, Leite DJ, Fursman M, Jeffrie N, Rahman IA, Philippe H, Copley RR, Telford MJ (2021) Lack of support for Deuterostomia prompts reinterpretation of the first Bilateria. Sci Adv 7:eabe2741
Kayal E, Bentlage B, Sabrina Pankey M, Ohdera AH, Medina M, Plachetzki DC, Collins AG, Ryan JF (2018) Phylogenomics provides a robust topology of the major cnidarian lineages and insights on the origins of key organismal traits. BMC Evol Biol 18:68
Kenny NJ, Francis WR, Rivera-Vicéns RE, Juarvel K, de Mendoza A, Díez-Vives C, Lister R, Bezares-Calderón LA, Grombacher L, Roller M, Barlow LD, Camilli S, Ryan JF, Wörheide G, Hill AL, Riesgo A, Leys SP (2020) Tracing animal genomic evolution with the chromosomal-level assembly of the freshwater sponge Ephydatia muelleri. Nat Commun 11:3676
Khalturin K, Shinzato C, Khalturina M, Hamada M, Fujie M, Koyanagi R, Kanda M, Goto H, Anton-Erxleben F, Toyokawa M, Toshino S, Satoh N (2019) Medusozoan genomes inform the evolution of the jellyfish body plan. Nat Ecol Evol 3:811–822
Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S (1997) The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124:4457–4466
Knabl P, Schauer A, Pomreinke AP, Zimmermann B, Rogers KW, Müller P, Genikhovich G (2024) Analysis of SMAD1/5 target genes in a sea anemone reveals ZSWIM4–6 as a novel BMP signaling modulator. eLife 13:e80803
Kraus C (2015) Phylum-wide transcriptome analysis of oogenesis and early embryogenesis in selected nematode species. Dissertation, Universität zu Köln. URL: https://kups.ub.uni-koeln.de/6746/. Accessed 26 Jan 2024
Kraus Y, Aman A, Technau U, Genikhovich G (2016) Pre-bilaterian origin of the blastoporal axial organizer. Nat Commun 7:11694
Kükenthal W (1925) Octocorallia. Walter de Gruyter, Berlin, Leipzig
Künnapuu J, Björkgren I, Shimmi O (2009) The Drosophila DPP signal is produced by cleavage of its proprotein at evolutionary diversified furin-recognition sites. Proc Natl Acad Sci U S A 106:8501–8506
Künnapuu J, Tauscher PM, Tiusanen N, Nguyen M, Löytynoja A, Arora K, Shimmi O (2014) Cleavage of the Drosophila screw prodomain is critical for a dynamic BMP morphogen gradient in embryogenesis. Dev Biol 389:149–159
Kuo DH, Weisblat DA (2011) A new molecular logic for BMP-mediated dorsoventral patterning in the leech Helobdella. Curr Biol 21:1282–1288
Lambert JD, Johnson AB, Hudson CN, Chan A (2016) Dpp/BMP2-4 Mediates Signaling from the D-Quadrant Organizer in a Spiralian Embryo. Curr Biol 26:2003–2010
Lanza AR, Seaver EC (2020) Functional evidence that Activin/Nodal signaling is required for establishing the dorsal-ventral axis in the annelid Capitella teleta. Development 147:dev189373
Lapraz F, Besnardeau L, Lepage T (2009) Patterning of the dorsal-ventral axis in echinoderms: insights into the evolution of the BMP-chordin signaling network. PLoS Biol 7:e1000248
Lapraz F, Haillot E, Lepage T (2015) A deuterostome origin of the Spemann organiser suggested by Nodal and ADMPs functions in Echinoderms. Nat Commun 6:8434
Le Petillon Y, Luxardi G, Scerbo P, Cibois M, Leon A, Subirana L, Irimia M, Kodjabachian L, Escriva H, Bertrand S (2017) Nodal/activin pathway is a conserved neural induction signal in chordates. Nat Ecol Evol 1:1192–1200
Lebedeva T, Aman AJ, Graf T, Niedermoser I, Zimmermann B, Kraus Y, Schatka M, Demilly A, Technau U, Genikhovich G (2021) Cnidarian-bilaterian comparison reveals the ancestral regulatory logic of the beta-catenin dependent axial patterning. Nat Commun 12:4032
Leclère L, Rentzsch F (2014) RGM regulates BMP-mediated secondary axis formation in the sea anemone Nematostella vectensis. Cell Rep 9:1921–1930
Leclère L, Horin C, Chevalier S, Lapebie P, Dru P, Peron S, Jager M, Condamine T, Pottin K, Romano S, Steger J, Sinigaglia C, Barreau C, Quiroga Artigas G, Ruggiero A, Fourrage C, Kraus JEM, Poulain J, Aury JM, Wincker P, Queinnec E, Technau U, Manuel M, Momose T, Houliston E, Copley RR (2019) The genome of the jellyfish Clytia hemisphaerica and the evolution of the cnidarian life-cycle. Nat Ecol Evol 3:801–810
Lee HX, Ambrosio AL, Reversade B, De Robertis EM (2006) Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell 124:147–159
Leininger S, Adamski M, Bergum B, Guder C, Liu J, Laplante M, Bråte J, Hoffmann F, Fortunato S, Jordal S, Rapp HT, Adamska M (2014) Developmental gene expression provides clues to relationships between sponge and eumetazoan body plans. Nat Comm 5(1):4905
Lele Z, Nowak M, Hammerschmidt M (2001) Zebrafish admp is required to restrict the size of the organizer and to promote posterior and ventral development. Dev Dyn 222:681–687
Little SC, Mullins MC (2009) Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol 11:637–643
Liu B, Ren X, Satou Y (2023) BMP signaling is required to form the anterior neural plate border in ascidian embryos. Dev Genes Evol 233:13–23
Lowe CJ, Terasaki M, Wu M, Freeman RM Jr, Runft L, Kwan K, Haigo S, Aronowicz J, Lander E, Gruber C, Smith M, Kirschner M, Gerhart J (2006) Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol 4:e291
Lu T-M, Luo Y-J, Yu J-K (2012) BMP and Delta/Notch signaling control the development of amphioxus epidermal sensory neurons: insights into the evolution of the peripheral sensory system. Development 139:2020–2030
Lyons KM, Hogan BL, Robertson EJ (1995) Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev 50:71–83
Lyons DC, Perry KJ, Batzel G, Henry JQ (2020) BMP signaling plays a role in anterior-neural/head development, but not organizer activity, in the gastropod Crepidula fornicata. Dev Biol 463:135–157
Marlow H, Matus DQ, Martindale MQ (2013) Ectopic activation of the canonical wnt signaling pathway affects ectodermal patterning along the primary axis during larval development in the anthozoan Nematostella vectensis. Dev Biol 380:324–334
Marqués G, Musacchio M, Shimell MJ, Wünnenberg-Stapleton K, Cho KW, O’Connor MB (1997) Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 91:417–426
Martin-Duran JM, Passamaneck YJ, Martindale MQ, Hejnol A (2016) The developmental basis for the recurrent evolution of deuterostomy and protostomy. Nat Ecol Evol 1:5
Martin-Zamora FM, Liang Y, Guynes K, Carrillo-Baltodano AM, Davies BE, Donnellan RD, Tan Y, Moggioli G, Seudre O, Tran M, Mortimer K, Luscombe NM, Hejnol A, Marletaz F, Martin-Duran JM (2023) Annelid functional genomics reveal the origins of bilaterian life cycles. Nature 615:105–110
Matus DQ, Pang K, Marlow H, Dunn CW, Thomsen GH, Martindale MQ (2006a) Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proc Natl Acad Sci U S A 103:11195–11200
Matus DQ, Thomsen GH, Martindale MQ (2006b) Dorso/ventral genes are asymmetrically expressed and involved in germ-layer demarcation during cnidarian gastrulation. Curr Biol 16:499–505
McFadden CS, Quattrini AM, Brugler MR, Cowman PF, Duenas LF, Kitahara MV, Paz-Garcia DA, Reimer JD, Rodriguez E (2021) Phylogenomics, Origin, and Diversification of Anthozoans (Phylum Cnidaria). Syst Biol 70:635–647
Meinhardt H (2015) Dorsoventral patterning by the Chordin-BMP pathway: a unified model from a pattern-formation perspective for Drosophila, vertebrates, sea urchins and Nematostella. Dev Biol 405:137–148
Miller DSJ, Schmierer B, Hill CS (2019) TGF-beta family ligands exhibit distinct signalling dynamics that are driven by receptor localisation. J Cell Sci 132:jcs234039
Miller-Bertoglio VE, Fisher S, Sanchez A, Mullins MC, Halpern ME (1997) Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol 192:537–550
Miyazawa K, Miyazono K (2017) Regulation of TGF-beta Family Signaling by Inhibitory Smads. Cold Spring Harb Perspect Biol 9:a0222095
Mizutani CM, Nie Q, Wan FY, Zhang YT, Vilmos P, Sousa-Neves R, Bier E, Marsh JL, Lander AD (2005) Formation of the BMP activity gradient in the Drosophila embryo. Dev Cell 8:915–924
Molina MD, Salo E, Cebria F (2007) The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev Biol 311:79–94
Molina MD, Neto A, Maeso I, Gomez-Skarmeta JL, Salo E, Cebria F (2011) Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr Biol 21:300–305
Moroz LL, Kocot KM, Citarella MR, Dosung S, Norekian TP, Povolotskaya IS, Grigorenko AP, Dailey C, Berezikov E, Buckley KM, Ptitsyn A, Reshetov D, Mukherjee K, Moroz TP, Bobkova Y, Yu F, Kapitonov VV, Jurka J, Bobkov YV, Swore JJ, Girardo DO, Fodor A, Gusev F, Sanford R, Bruders R, Kittler E, Mills CE, Rast JP, Derelle R, Solovyev VV, Kondrashov FA, Swalla BJ, Sweedler JV, Rogaev EI, Halanych KM, Kohn AB (2014) The ctenophore genome and the evolutionary origins of neural systems. Nature 510:109–114
Mulhair PO, McCarthy CGP, Siu-Ting K, Creevey CJ, O’Connell MJ (2022) Filtering artifactual signal increases support for Xenacoelomorpha and Ambulacraria sister relationship in the animal tree of life. Curr Biol 32(5180–5188):e5183
Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Nusslein-Volhard C (1996) Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development 123:81–93
Nichols SA, Roberts BW, Richter DJ, Fairclough SR, King N (2012) Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/beta-catenin complex. Proc Natl Acad Sci U S A 109:13046–13051
Nielsen C, Brunet T, Arendt D (2018) Evolution of the bilaterian mouth and anus. Nat Ecol Evol 2:1358–1376
Nishimatsu S, Thomsen GH (1998) Ventral mesoderm induction and patterning by bone morphogenetic protein heterodimers in Xenopus embryos. Mech Dev 74:75–88
Nunes da Fonseca R, von Levetzow C, Kalscheuer P, Basal A, van der Zee M, Roth S (2008) Self-regulatory circuits in dorsoventral axis formation of the short-germ beetle Tribolium castaneum. Dev Cell 14:605–615
Oelgeschlager M, Larrain J, Geissert D, De Robertis EM (2000) The evolutionarily conserved BMP-binding protein twisted gastrulation promotes BMP signalling. Nature 405:757–763
Ogawa K, Ishihara S, Saito Y, Mineta K, Nakazawa M, Ikeo K, Gojobori T, Watanabe K, Agata K (2002) Induction of a noggin-like gene by ectopic DV interaction during planarian regeneration. Dev Biol 250:59–70
Ohta N, Satou Y (2013) Multiple signaling pathways coordinate to induce a threshold response in a chordate embryo. PLoS Genet 9:e1003818
Onai T, Yu JK, Blitz IL, Cho KW, Holland LZ (2010) Opposing Nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. Dev Biol 344:377–389
Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C (1999) Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature 401:480–485
Orii H, Watanabe K (2007) Bone morphogenetic protein is required for dorso-ventral patterning in the planarian Dugesia japonica. Dev Growth Differ 49:345–349
Özüak O, Buchta T, Roth S, Lynch JA (2014) Dorsoventral polarity of the Nasonia embryo primarily relies on a BMP gradient formed without input from Toll. Curr Biol 24:2393–2398
Pang K, Ryan JF, Baxevanis AD, Martindale MQ (2011) Evolution of the TGF-beta signaling pathway and its potential role in the ctenophore. Mnemiopsis Leidyi Plos One 6:e24152
Patterson GI, Padgett RW (2000) TGF beta-related pathways. Roles in Caenorhabditis elegans development. Trends Genet 16:27–33
Pax F (1925) Hexacorallia. Walter de Gruyter, Berlin, Leipzig
Pechmann M, Kenny NJ, Pott L, Heger P, Chen YT, Buchta T, Ozuak O, Lynch JA, Roth S (2021) Striking parallels between dorsoventral patterning in Drosophila and Gryllus reveal a complex evolutionary history behind a model gene regulatory network. Elife 10:e68287
Peluso CE, Umulis D, Kim YJ, O’Connor MB, Serpe M (2011) Shaping BMP morphogen gradients through enzyme-substrate interactions. Dev Cell 21:375–383
Piccolo S, Sasai Y, Lu B, De Robertis EM (1996) Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86:589–598
Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM (1997) Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell 91:407–416
Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM (1999) The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature 397:707–710
Pisani D, Pett W, Dohrmann M, Feuda R, Rota-Stabelli O, Philippe H, Lartillot N, Wörheide G (2015) Genomic data do not support comb jellies as the sister group to all other animals. Proc Natl Acad Sci USA 112:15402–15407
Ploper D, Lee HX, De Robertis EM (2011) Dorsal-ventral patterning: Crescent is a dorsally secreted Frizzled-related protein that competitively inhibits Tolloid proteases. Dev Biol 352:317–328
Plouhinec JL, Zakin L, Moriyama Y, De Robertis EM (2013) Chordin forms a self-organizing morphogen gradient in the extracellular space between ectoderm and mesoderm in the Xenopus embryo. Proc Natl Acad Sci U S A 110:20372–20379
Pomreinke AP, Soh GH, Rogers KW, Bergmann JK, Bläßle AJ, Müller P (2017) Dynamics of BMP signaling and distribution during zebrafish dorsal-ventral patterning. Elife 6:e25861
Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, Jurka J, Genikhovich G, Grigoriev IV, Lucas SM, Steele RE, Finnerty JR, Technau U, Martindale MQ, Rokhsar DS (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317:86–94
Ramel MC, Hill CS (2013) The ventral to dorsal BMP activity gradient in the early zebrafish embryo is determined by graded expression of BMP ligands. Dev Biol 378:170–182
Reber-Müller S, Streitwolf-Engel R, Yanze N, Schmid V, Stierwald M, Erb M, Seipel K (2006) BMP2/4 and BMP5-8 in jellyfish development and transdifferentiation. Int J Dev Biol 50:377–384
Reddien PW, Bermange AL, Kicza AM, Sanchez Alvarado A (2007) BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development 134:4043–4051
Reinhardt B, Broun M, Blitz IL, Bode HR (2004) HyBMP5-8b, a BMP5-8 orthologue, acts during axial patterning and tentacle formation in hydra. Dev Biol 267:43–59
Remane A (1950) Die Entstehung der Metamerie der Wirbellosen. Verh Deutsch Zool Ges 14:16–23
Rentzsch F, Anton R, Saina M, Hammerschmidt M, Holstein TW, Technau U (2006) Asymmetric expression of the BMP antagonists chordin and gremlin in the sea anemone Nematostella vectensis: implications for the evolution of axial patterning. Dev Biol 296:375–387
Reversade B, De Robertis EM (2005) Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123:1147–1160
Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM (2005) Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development 132:3381–3392
Rivron NC, Frias-Aldeguer J, Vrij EJ, Boisset JC, Korving J, Vivie J, Truckenmuller RK, van Oudenaarden A, van Blitterswijk CA, Geijsen N (2018) Blastocyst-like structures generated solely from stem cells. Nature 557:106–111
Rogers KW, ElGamacy M, Jordan BM, Müller P (2020) Optogenetic investigation of BMP target gene expression diversity. Elife 9:e58641
Romanova-Michaelides M, Hadjivasiliou Z, Aguilar-Hidalgo D, Basagiannis D, Seum C, Dubois M, Julicher F, Gonzalez-Gaitan M (2022) Morphogen gradient scaling by recycling of intracellular Dpp. Nature 602:287–293
Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O’Connor MB, Marsh JL (2001) Twisted gastrulation is a conserved extracellular BMP antagonist. Nature 410:479–483
Roth S (2023) Neofunctionalization of Toll Signaling in Insects: From Immunity to Dorsoventral Patterning. Annu Rev Cell Dev Biol 39:1–22
Röttinger E, Martindale MQ (2011) Ventralization of an indirect developing hemichordate by NiCl(2) suggests a conserved mechanism of dorso-ventral (D/V) patterning in Ambulacraria (hemichordates and echinoderms). Dev Biol 354:173–190
Röttinger E, Dahlin P, Martindale MQ (2012) A framework for the establishment of a cnidarian gene regulatory network for “endomesoderm” specification: the inputs of ss-catenin/TCF signaling. PLoS Genet 8:e1003164
Röttinger E, DuBuc TQ, Amiel AR, Martindale MQ (2015) Nodal signaling is required for mesodermal and ventral but not for dorsal fates in the indirect developing hemichordate, Ptychodera flava. Biol Open 4:830–842
Roure A, Chowdhury R, Darras S (2023) Regulation of anterior neurectoderm specification and differentiation by BMP signaling in ascidians. Development 150:dev201575
Ryan JF, Mazza ME, Pang K, Matus DQ, Baxevanis AD, Martindale MQ, Finnerty JR (2007) Pre-bilaterian origins of the Hox cluster and the Hox code: evidence from the sea anemone. Nematostella Vectensis Plos One 2:e153
Ryan JF, Pang K, Schnitzler CE, Nguyen AD, Moreland RT, Simmons DK, Koch BJ, Francis WR, Havlak P, Program NCS, Smith SA, Putnam NH, Haddock SH, Dunn CW, Wolfsberg TG, Mullikin JC, Martindale MQ, Baxevanis AD (2013) The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science 342:1242592
Sachs L, Chen YT, Drechsler A, Lynch JA, Panfilio KA, Lässig M, Berg J, Roth S (2015) Dynamic BMP signaling polarized by Toll patterns the dorsoventral axis in a hemimetabolous insect. Elife 4:e05502
Sagner A, Briscoe J (2019) Establishing neuronal diversity in the spinal cord: a time and a place. Development 146:dev182154
Saina M, Genikhovich G, Renfer E, Technau U (2009) BMPs and chordin regulate patterning of the directive axis in a sea anemone. Proc Natl Acad Sci U S A 106:18592–18597
Santini S, Schenkelaars Q, Jourda C, Duchesne M, Belahbib H, Rocher C, Selva M, Riesgo A, Vervoort M, Leys SP, Kodjabachian L, Le Bivic A, Borchiellini C, Claverie J-M, Renard E (2023) The compact genome of the sponge Oopsacas minuta (Hexactinellida) is lacking key metazoan core genes. BMC Biol 21:139
Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM (1994) Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79:779–790
Schmid B, Furthauer M, Connors SA, Trout J, Thisse B, Thisse C, Mullins MC (2000) Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development 127:957–967
Schultz DT, Haddock SHD, Bredeson JV, Green RE, Simakov O, Rokhsar DS (2023) Ancient gene linkages support ctenophores as sister to other animals. Nature 618:110–117
Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KW, Greenspan DS (1999) Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol 213:283–300
Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KW, Greenspan DS (2001) Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature 410:475–478
Sedgwick A (1884) On the origin of metameric segmentation and some other morphological questions. Quart J Microsc Sci 93:43–82
Shen Q, Toulabi LB, Shi H, Nicklow EE, Liu J (2018) The forkhead transcription factor UNC-130/FOXD integrates both BMP and Notch signaling to regulate dorsoventral patterning of the C. elegans postembryonic mesoderm. Dev Biol 433:75–83
Shimmi O, O’Connor MB (2003) Physical properties of Tld, Sog, Tsg and Dpp protein interactions are predicted to help create a sharp boundary in Bmp signals during dorsoventral patterning of the Drosophila embryo. Development 130:4673–4682
Shimmi O, Umulis D, Othmer H, O’Connor MB (2005) Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120:873–886
Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M, Fujie M, Fujiwara M, Koyanagi R, Ikuta T, Fujiyama A, Miller DJ, Satoh N (2011) Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 476:320–323
Simion P, Philippe H, Baurain D, Jager M, Richter DJ, Di Franco A, Roure B, Satoh N, Queinnec E, Ereskovsky A, Lapebie P, Corre E, Delsuc F, King N, Wörheide G, Manuel M (2017) A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals. Curr Biol 27:958–967
Simon N, Safyan A, Pyrowolakis G, Matsuda S (2023) Dally is not essential for Dpp spreading or internalization but for Dpp stability by antagonizing Tkv-mediated Dpp internalization. eLife 12:RP86663
Solloway MJ, Robertson EJ (1999) Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development 126:1753–1768
Sopory S, Kwon S, Wehrli M, Christian JL (2010) Regulation of Dpp activity by tissue-specific cleavage of an upstream site within the prodomain. Dev Biol 346:102–112
Soukup V, Kozmik Z (2018) The Bmp signaling pathway regulates development of left-right asymmetry in amphioxus. Dev Biol 434:164–174
Spemann H, Mangold H (1924) Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Archiv Für Mikroskopische Anatomie Und Entwicklungsmechanik 100:599–638
Srinivasan S, Rashka KE, Bier E (2002) Creation of a Sog morphogen gradient in the Drosophila embryo. Dev Cell 2:91–101
Srivastava M, Simakov O, Chapman J et al (2010) The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 466:720–726
Srivastava M, Mazza-Curll KL, van Wolfswinkel JC, Reddien PW (2014) Whole-body acoel regeneration is controlled by Wnt and Bmp-Admp signaling. Curr Biol 24:1107–1113
Stephenson TA (1928) The British Sea Anemones by T. A. Stephenson. Ray Society, United Kingdom
Su YH, Chen YC, Ting HC, Fan TP, Lin CY, Wang KT, Yu JK (2019) BMP controls dorsoventral and neural patterning in indirect-developing hemichordates providing insight into a possible origin of chordates. Proc Natl Acad Sci USA 116:12925–12932
Suzuki A, Kaneko E, Maeda J, Ueno N (1997) Mesoderm induction by BMP-4 and -7 heterodimers. Biochem Biophys Res Commun 232:153–156
Suzuki Y, Yandell MD, Roy PJ, Krishna S, Savage-Dunn C, Ross RM, Padgett RW, Wood WB (1999) A BMP homolog acts as a dose-dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development 126:241–250
Tajer B, Dutko JA, Little SC, Mullins MC (2021) BMP heterodimers signal via distinct type I receptor class functions. Proc Natl Acad Sci USA 118:e2017952118
Tan S, Huan P, Liu B (2017) Expression patterns indicate that BMP2/4 and Chordin, not BMP5-8 and Gremlin, mediate dorsal-ventral patterning in the mollusk Crassostrea gigas. Dev Genes Evol 227:75–84
Tan S, Huan P, Liu B (2018) An investigation of oyster TGF-beta receptor genes and their potential roles in early molluscan development. Gene 663:65–71
Tan S, Huan P, Liu B (2022) Molluskan dorsal-ventral patterning relying on BMP2/4 and chordin provides insights into spiralian development and evolution. Mol Biol Evol 39:msab322
Technau U, Genikhovich G (2018) Evolution: directives from sea anemone Hox genes. Curr Biol 28:R1303–R1305
Tuazon FB, Wang X, Andrade JL, Umulis D, Mullins MC (2020) Proteolytic restriction of Chordin range underlies BMP gradient formation. Cell Rep 32:108039
Tucker JA, Mintzer KA, Mullins MC (2008) The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell 14:108–119
van Beneden E (1891) Recherches sur le développement des Arachnactis. Contribution a la morphologie de Cérianthides. Arch Biol Paris 11:115–146
van der Zee M, Stockhammer O, von Levetzow C, Nunes da Fonseca R, Roth S (2006) Sog/Chordin is required for ventral-to-dorsal Dpp/BMP transport and head formation in a short germ insect. Proc Natl Acad Sci U S A 103:16307–16312
Waki K, Imai KS, Satou Y (2015) Genetic pathways for differentiation of the peripheral nervous system in ascidians. Nat Commun 6:8719
Wang YC, Ferguson EL (2005) Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature 434:229–234
Watanabe H, Schmidt HA, Kuhn A, Hoger SK, Kocagoz Y, Laumann-Lipp N, Ozbek S, Holstein TW (2014) Nodal signalling determines biradial asymmetry in Hydra. Nature 515:112–115
Webster NB, Corbet M, Sur A, Meyer NP (2021) Role of BMP signaling during early development of the annelid Capitella teleta. Dev Biol 478:183–204
Weiss JB, Von Ohlen T, Mellerick DM, Dressler G, Doe CQ, Scott MP (1998) Dorsoventral patterning in the Drosophila central nervous system: the intermediate neuroblasts defective homeobox gene specifies intermediate column identity. Genes Dev 12:3591–3602
Wharton SJ, Basu SP, Ashe HL (2004) Smad affinity can direct distinct readouts of the embryonic extracellular Dpp gradient in Drosophila. Curr Biol 14:1550–1558
Whelan NV, Kocot KM, Moroz LL, Halanych KM (2015) Error, signal, and the placement of Ctenophora sister to all other animals. Proc Natl Acad Sci U S A 112:5773–5778
Winnier G, Blessing M, Labosky PA, Hogan BL (1995) Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9:2105–2116
Xue Y, Zheng X, Huang L, Xu P, Ma Y, Min Z, Tao Q, Tao Y, Meng A (2014) Organizer-derived Bmp2 is required for the formation of a correct Bmp activity gradient during embryonic development. Nat Commun 5:3766
Yan Y, Ning G, Li L, Liu J, Yang S, Cao Y, Wang Q (2019) The BMP ligand Pinhead together with Admp supports the robustness of embryonic patterning. Sci Adv 5:eaau6455
Yu JK, Satou Y, Holland ND, Shin IT, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ (2007) Axial patterning in cephalochordates and the evolution of the organizer. Nature 445:613–617
Zapata F, Goetz FE, Smith SA, Howison M, Siebert S, Church SH, Sanders SM, Ames CL, McFadden CS, France SC, Daly M, Collins AG, Haddock SH, Dunn CW, Cartwright P (2015) Phylogenomic analyses support traditional relationships within Cnidaria. PLoS ONE 10:e0139068
Zhu R, Santat LA, Markson JS, Nandagopal N, Gregrowicz J, Elowitz MB (2023) Reconstitution of morphogen shuttling circuits. Sci Adv 9:eadf9336
Zimmerman LB, De Jesus-Escobar JM, Harland RM (1996) The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86:599–606
Zimmermann B, Montenegro JD, Robb SMC, Fropf WJ, Weilguny L, He S, Chen S, Lovegrove-Walsh J, Hill EM, Chen C-Y, Ragkousi K, Praher D, Fredman D, Schultz D, Moran Y, Simakov O, Genikhovich G, Gibson MC, Technau U (2023) Topological structures and syntenic conservation in sea anemone genomes. Nat Comm 14:8270
Zinski J, Bu Y, Wang X, Dou W, Umulis D, Mullins MC (2017) Systems biology derived source-sink mechanism of BMP gradient formation. Elife 6:e22199
Funding
Open access funding provided by University of Vienna. The work in the Genikhovich group is funded by the Austrian Science Foundation (FWF) (grants P32705-B and P36080-B). David Mörsdorf is a recipient of the Lise Meitner Fellowship (grant M3291-B) of the Austrian Science Foundation (FWF).
Author information
Authors and Affiliations
Contributions
D.M. and G.G. wrote the manuscript. D.M., P.K., and G.G. prepared the figures and edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Kristen Panfilio.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mörsdorf, D., Knabl, P. & Genikhovich, G. Highly conserved and extremely evolvable: BMP signalling in secondary axis patterning of Cnidaria and Bilateria. Dev Genes Evol (2024). https://doi.org/10.1007/s00427-024-00714-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00427-024-00714-4