Abstract
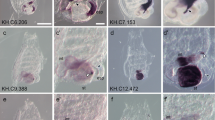
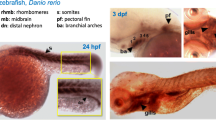
In vertebrates, glycine amidinotransferase (AGAT), guanidinoacetate methyltransferase (GAMT), and creatine transporter (CT1) are three proteins involved in creatine synthesis. To provide insight into the molecular evolution mechanism of creatine metabolism, we have cloned and identified BbAGAT, BbGAMT, and BbCT1 homologous genes in amphioxus (Branchiostoma belcheri), whose predicted proteins show high identities with AGAT, GAMT, and CT1 proteins in vertebrates. The phylogenetic analysis indicates that amphioxus AGAT, GAMT, and CT1 are branched off at the base of the vertebrate homologous clade, respectively. Genomic structures of BfAGAT, BfGAMT, and BfCT1 show their comparability with the homologs in vertebrate and original characteristic of cephalochordate, which is consistent with animal classification. To determine the expression patterns of BbAGAT, BbGAMT, and BbCT1, whole-mount and section in situ hybridizations are carried out in embryos and adults of amphioxus. During embryogenesis, they are all expressed mainly in mesendoderm and late somites, but BbCT1 is also expressed in differentiating notochord and digestive tract, as well as in the cytoplasm of zygotes and the blastomeres at cleavage stage. In adult, the transcripts of BbAGAT and BbGAMT are detected in the neural cord, gill, nephridia, endostyle, gut, and gonads, while BbCT1 is expressed mainly in the epithelium of gut. The expression pattern of these three genes is similar to their vertebrate homologs. The result reveals that AGAT, GAMT, and CT1, the primary elements of vertebrate creatine metabolism, exist in cephalochordate amphioxus, and are highly conserved during evolution. It also suggests that similar mechanism of creatine synthesis in vertebrate may occur in amphioxus.




Similar content being viewed by others
References
Almeida LS, Verhoeven NM, Roos B, Valongo C, Cardoso ML, Vilarinho L, Salomons GS, Jakobs C (2004) Creatine and guanidinoacetate: diagnostic markers for inborn errors in creatine biosynthesis and transport. Mol Genet Metab 82:214–219
Battini R, Alessandri MG, Leuzzi V, Moro F, Tosetti M, Bianchi MC, Cioni G (2006) Arginine:glycine amidinotransferase (AGAT) deficiency in a newborn: early treatment can prevent phenotypic expression of the disease. J Pediatr 148:828–830
Braissant O, Henry H, Villard AM, Speer O, Wallimann T, Bachmann C (2005) Creatine synthesis and transport during rat embryogenesis: spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev Biol 5:9
Chae YJ, Chung CE, Kim BJ, Lee MH, Lee H (1998) The gene encoding guanidinoacetate methyltransferase (GAMT) maps to human chromosome 19 at band p13.3 and to mouse chromosome 10. Genomics 49:162–164
DeLigio JT, Ellington WR (2006) The capacity for the de novo biosynthesis of creatine is present in the tunicate Ciona intestinalis and is likely widespread in other protochordate and invertebrate groups. Comp Biochem Physiol Part D Genomics Proteomics 1:167–178
Ellington WR, Suzuki T (2007) Early evolution of the creatine kinase gene family and the capacity for creatine biosynthesis and membrance transport. In: Salomons G, Wyss M (eds) Creatine and creatine kinase in health and disease. Springer Verlag-Subcellular Biochemistry Series
Fang YQ, Wang H, Zhang CL (1991) Correlation of annual change of luteinizing hormone–releasing hormone (LH–RH) with gonadal development in amphioxus. Sci China B 34:814–822
Graber NA, Ellington WR (2001) Gene duplication events producing muscle (M) and brain (B) isoforms of cytoplasmic creatine kinase: cDNA and deduced amino acid sequences from two lower chordates. Mol Biol Evol 18:1305–1314
Holland PW (1999) Wholemount in situ hybridization to amphioxus embryos. Methods Mol Biol 97:641–644
Isbrandt D, von Figura K (1995) Cloning and sequence analysis of human guanidinoacetate N-methyltransferase cDNA. Biochim Biophys Acta 1264:265–267
Lee H, Ogawa H, Fujioka M, Gerton GL (1994) Guanidinoacetate methyltransferase in the mouse: extensive expression in Sertoli cells of testis and in microvilli of caput epididymis. Biol Reprod 50:152–162
Lee H, Kim JH, Chae YJ, Ogawa H, Lee MH, Gerton GL (1998) Creatine synthesis and transport systems in the male rat reproductive tract. Biol Reprod 58:1437–1444
Mao B, Zhang S, Wu X, Zhang H (1997) Morphological and functional studies on the epidermal cells of amphioxus at different developmental stages. Chin J Oceanol Limnol 15:236–240
Robin Y (1964) Biological distribution of guanidines and phosphagens in marine annelida and related phyla from California, with a note on pluriphosphagens. Comp Biochem Physiol 12:347–367
Roche J, Nguyen-Van T, Robin Y (1957) [Presence of creatine in invertebrates, and its biological significance.]. Biochim Biophys Acta 24:514–519
Salomons GS, Wyss M (2007) Creatine and creatine kinase in health and disease. Springer, Dordrecht
Salomons GS, van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, Degrauw TJ, Jakobs C (2001) X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet 68:1497–1500
Schulze A (2003) Creatine deficiency syndromes. Mol Cell Biochem 244:143–150
Schulze A, Bachert P, Schlemmer H, Harting I, Polster T, Salomons GS, Verhoeven NM, Jakobs C, Fowler B, Hoffmann GF, Mayatepek E (2003) Lack of creatine in muscle and brain in an adult with GAMT deficiency. Ann Neurol 53:248–251
Stromberger C, Bodamer OA, Stockler-Ipsiroglu S (2003) Clinical characteristics and diagnostic clues in inborn errors of creatine metabolism. J Inherit Metab Dis 26:299–308
Sykut-Cegielska J, Gradowska W, Mercimek-Mahmutoglu S, Stockler-Ipsiroglu S (2004) Biochemical and clinical characteristics of creatine deficiency syndromes. Acta Biochim Pol 51:875–882
Torremans A, Marescau B, Possemiers I, Van Dam D, D’Hooge R, Isbrandt D, De Deyn PP (2005) Biochemical and behavioural phenotyping of a mouse model for GAMT deficiency. J Neurol Sci 231:49–55
van der Knaap MS VN, Maaswinkel-Mooij P, Pouwels PJ, Onkenhout W, Peeters EA, Stkler-Ipsiroglu S, Jakobs C (2000) Mental retardation and behavioral problems as presenting signs of a creatine synthesis defect. Ann Neurol 47:540–543
Van Pilsum JF, Stephens GC, Taylor D (1972) Distribution of creatine, guanidinoacetate and enzymes for their biosynthesis in the animal kingdom. Implications for phylogeny. Biochem J 126:325–345
Van Pilsum JF, Taylor D, Bans L (1975) Studies of the uptake of creatine from sea water by the marine annelid, Glycera dibranchiata. Comp Biochem Physiol 51:611–617
Wang L, Zhang Y, Shao M, Zhang H (2007) Spatiotemporal expression of the creatine metabolism related genes agat, gamt and ct1 during zebrafish embryogenesis. Int J Dev Biol 51:247–253
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213
Zhang H, Huang Z, Yamaguchi K, Tomonaga S (1992) Granulocytes and macrophages in amphioxus. Zool Sci 9:113–118
Zhao H, Cao Y, Grunz H (2001) Expression of Xenopus l-arginine:glycine amidinotransferase (XAT) during early embryonic development. Dev Genes Evol 211:358–360
Acknowledgements
We thank Dr. Jeremy Gibson-Brown for the copyediting suggestions of the manuscript. This project was funded by grants from CNSF (30270693, 30570967) and the Ministry of Science and Technology of China (2007CB947100, 2007CB815800).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Gibson-Brown
Lifeng Wang and Dongyan Chen contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material
ESM 1
(GIF 121 KB)
Rights and permissions
About this article
Cite this article
Wang, L., Chen, D., Zhang, Y. et al. Characterization of AGAT, GAMT and CT1 in amphioxus: implications for the evolutionary conservation of creatine metabolism related molecules at the invertebrate-to-vertebrate transition. Dev Genes Evol 218, 681–689 (2008). https://doi.org/10.1007/s00427-008-0241-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-008-0241-0