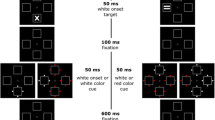
Abstract
In peripheral spatial cueing paradigms, exogenous attentional capture is commonly observed after salient onset cues or with cues contingent on target characteristics. We proposed that exogenously captured attention disrupts the selectivity to target features. We tested this by experimentally emulating the everyday observation that in a viewing situation in which the observer is monitoring a stationary display fort change to occur, the onset of a salient stimulus (onset cue) or a change in a stationary stimulus similar to the expected one (contingent cue) has a distracting effect. As predicted, we found that both types of cues reduced the target detection sensitivity but enhanced the bias to respond in a go-nogo-paradigm. With the onset cue, the sensitivity loss was more pronounced at the side of the cue, whereas the contingent cue affected both sides likewise. Moreover, the effects of the onset cue interacted with the task difficulty: the more selectivity a task required the more immune it was against disruption, but the more likely was a response. We concluded that onset capture disrupts selective attention by adding noise to the processing of the target location. The effects of contingent capture could be explained with cue-target confounding. Finally, we suggest a new model of attentional capture in which exogenous and endogenous components interact in a dynamic way.





Similar content being viewed by others
Notes
The distinction between the mechanisms of channel enhancement and selection is based on a paper by the same first author with the promising title ‘Phenomenology of Attention’ (Prinzmetal, H., Allen, & Edwards, 1998), which, however, might be criticized on several grounds: First, although the majority of experiments in the paper deals with the question of how attention alters the appearance of a color dot, the authors manipulated attention to letters at a location close to the colored dot, and not attention to a specific color or color contrast itself. Had they done this, the appearance of the dot might have changed. Second, they measured appearance by having the subjects match the color of the dot to a color on a test palette. However, by doing this, the subject most plausibly paid attention to the colors of the palette, thereby—if attention altered appearance—it most likely changed the appearance of the test colors as well, thereby canceling out the effect. So it is not surprising that almost no hue shift was observed between ‘attended’ and ‘unattended’ conditions. Third, the colors in the test palette were presented in the context of other colors which might also have changed their appearance (e.g., Ekroll, Faul, & Niederée, 2004).
Several papers in the literature have shown that the discrimination of weak color differences is improved by and necessitates the allocation of voluntary attention: For example, Corbetta, Miezin, Dobmeyer, Shulman, and Petersen (1991) found that nonspatial attention to color enhanced the accuracy to detect near-threshold changes. Just recently, Jehee, Brady, and Tong (2011) published evidence from a spatial cueing paradigm that voluntary attention to color on either side of the display enhanced the accuracy to detect a near-threshold color change at the cued side (but see Andersen, Müller, & Hillyard, 2009 for the claim that attention to features can also operate location-independently). Nagy, Sanchez, and Hughes (1990) used visual search to demonstrate the effect of voluntary attention on detecting small color differences in foveal as well as peripheral vision.
Bashinsky and Bacharach (1980) as well as as Müller and Findley (1987) employed a detection plus localization task to circumvent this problem: If a subject responded ‘yes’, he/she was asked to indicate the perceived target position. By relating the cue position to this locational response, the erroneously detected target in a false alarm could be classified as being either validly or invalidly cued. However, beside the question of how these data are analyzed correctly (see Appendix A of Müller & Findley, 1987), in a detection task two conceptual problems arise: First, an objective cue validity (in trials with a target) is confused with a subjective one (in trials without a target), allowing for the possibility that, if a target is erroneously perceived at the nontarget side, the cue is subjectively valid but objectively invalid. Second, it is most likely that performing a detection plus localization task profoundly alters the operation mode of selective attention, compared to detection alone.
Prinzmetal et al. (2008, exp. 3) proceeded differently: At each location, they independently presented either a target or not, and had the subjects judge target presence separately for the two locations. Although this is a nice solution because it allows to assign false alarms to cued and uncued locations, we did not find it suitable for our research question: First, since either 0, 1, or 2 targets could appear, the task is quite complex from the beginning, thus the attentional control set is broader and selectivity less pronounced. Second, target presence at one location might act like an additional 0 ms-SOA-cue for the other one. Third (although implicitly) the task is detection plus localization again. Finally, the procedure is not suited to test the alertness hypothesis, i.e., the effect of the cue on the bias to respond.
Liu et al. (2005) used a so-called response cue at the end of each trial, telling the subject which stimulus position to respond to. Besides this procedure seeming more elegant than the double response of Müller and Findley (1987), it poses the same problems when applied to a detection task. Moreover, it is not suitable for investigating the alertness hypothesis again.
Note that this is different from the ROC plots in Fig. 2 where the SDT model had been fitted to the pooled data of all subjects. Generally, the SDT with averaged parameter estimates does not provide a good fit to the pooled data. Figure 4 should illustrate the hypotheses tests, which are carried out on the mean parameter estimates, whereas Fig. 2 should illustrate the model fit. Note further that all model fits were carried out with the constraint of equidistant criteria λlib, λbal, and λcon for cued and uncued trials.
References
Allport, D. A. (1987). Selection for action: Some behavioral and neurophysiological consideration of attention and action. In H. Heuer & A. F. Sanders (Eds.), Perspectives on perception and action. Hillsdale, NJ: Erlbaum.
Andersen, S. K., Müller, M. M., Hillyard, S. A. (2009). Color-selective attention need not be mediated by spatial attention. Journal of Vision, 9(6), 1–7.
Ansorge, U., Heumann, M. (2003). Top-down contingencies in peripheral cuing: The roles of color and location. Journal of Experimental Psychology: Human Perception & Performance, 29(5), 937–948.
Awh, E., Belopolsky, A., Theeuwes, J. (2012). Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends in Cognitive Sciences, 16(8), 437–443.
Bashinsky, H. S., Bacharach, V. R. (1980). Enhancement of perceptual sensitivity as the result of selectively attending to spatial locations. Perception & Psychophysics, 28, 241–248.
Benjamin, A. S., Diaz, M. L., Wee, S. (2009). Signal detection with criterion noise: Applications to recognition memory. Psychological Review, 116, 84–114.
Beringer, J. (1987). Experimental Run Time System (ERTS): Software for developing and running reaction time experiments on IBM-compatibe PCs (Version v3.31). Frankfurt/M: BeriSoft Cooperation.
Bonnel, A. M., Miller, J (1994). Attentional effects on concurrent discriminations: Investigations of a sample-size model. Perception and Psychophysics, 54, 162–179.
Bradley, J. (1968). Distribution-free statistical tests. Englewood Cliffs: Prentice-Hall.
Carrasco, M., Ling, S., Read, S. (2004). Attention alters appearance. Nature Neuroscience, 7, 308–313.
Carrasco, M., Penpeci-Talgar, C., Eckstein, M. (2000). Spatial covert attention increases contrast sensitivity across the CSF: support for signal enhancement. Vision Research, 40, 1203–1215.
Castiello, U., Umiltà, C. (1990). Size of the attentional focus and efficiency of processing. Acta Psychologica, 73, 195–209.
Chastain, G. (1992). Is rapid performance improvement across short precue-target delays due to masking from peripheral precues? Acta Psychologica, 79, 101–114.
Corbetta, M., Miezin, F. M., Dobmeyer, S., Shulman, G. L., Petersen, S. E. (1991). Selective and divided attention during visual discriminations of shape, color, and speed: functional anatomy by positron emission tomography. The Journal of Neuroscience, 11(8), 2383–2401.
Cosman, J. D., Vecera, S. P. (2009). Perceptual load modulates attentional capture by abrupt onsets. Psychonomic Bulletin & Review, 16, 404–410.
Danilova, M. V., Mollon, J. D. (2011). Parafoveal color discrimination: a chromaticity locus of enhanced discrimination. Journal of Vision, 10(1), 1–9.
Eisner, A., MacLeod, D. I. A. (1980). Blue-sensitive cones do not contribute to luminance. Journal of the Optical Society of America, 70(1), 121–123.
Ekroll, V., Faul, F., Niederée, R. (2004). The peculiar nature of simultaneous colour contrast in uniform surrounds. Vision Research, 44, 1765–1786.
Eriksen, C. W., St. James, J. D. (1986). Visual attention within and around the field of focal attention: A zoom lens model. Perception & Psychophysics, 40(4), 225–240.
Facoetti, A., Corradi, N., Ruffino, M., Gori, S., Zorzi, M. (2010). Multisensory spatial attention deficits are predictive of phonological decoding skills in developmental dyslexia. Dyslexia, 22(5), 1011–1025.
Facoetti, A., Molteni, M. (2001). The gradient of visual attention in developmental dyslexia. Neuropsychologia, 39, 352–357.
Facoetti, A., Trussardi, A. N., Ruffino, M., Lorusso, M. L., Cattaneo, C., Galli, R., Zorzi, M. (2010). Multisensory spatial attention deficits are predictive of phonological decoding skills in developmental dyslexia. Journal of Cognitive Neuroscience, 22(5), 1011–1025.
Folk, C. L., Remington, R. W., Johnston, J. C. (1992). Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance, 18(4), 1030–1044.
Franceschini, S., Gori, S., Ruffino, M., Pedrolli, K., Facoetti, A. (2012). A causal link between visual spatial attention and reading acquisition. Current Biology, 22, 1–6.
Fuller, S., Carrasco, M. (2006). Exogenous attention and color perception: Performance and appearance of saturation and hue. Vision Research, 46, 4032–4047.
Gilbert, M. (1950). Colour perception in parafoveal vision. Proceedings of the Physical Society London, 63, 83–89.
Gould, I. C., Wolfgang, B. W., Smith, P. L. (2007). Spatial uncertainty explains exogenous and endogenous attentional cuing effects in visual signal detection. Journal of Vision, 7(4), 1–17.
Green, D. M., Swets, J. A. (1966). Signal Detection Theory and Psychophysics. New York: Wiley.
Henderson, J. M. (1991). Stimulus discrimination following covert attentional orienting to an exogenous cue. Journal of Experimental Psychology: Human Perception and Performance, 17, 91–106.
Henderson, J. M., Macquistan, A. (1993). The spatial distribution of attention following an exogenous cue. Perception and Psychophysics, 53(2), 221–230.
James, W. (1950). The principles of psychology (Vol. 1). New York: Dover (Original work published 1890).
Jehee, J. F. M., Brady, D. K., Tong, F. (2011). Attention improves encoding of task-relevant features in the human visual cortex. The Journal of Neuroscience, 31(22), 8210–8219.
Jonides, J. (1981). Voluntary vs. automatic control of the mind’s eye’s movement. In J. B. Long & A. Baddeley (Eds.), Attention and performance IX (pp. 187–204). Hillsdale: Erlbaum.
Kerzel, D., Zarian, L., Suoto, D. (2009). Involuntary cueing effects on accuracy measures: Stimulus and task dependence. Journal of Vision, 9(11), 1–16.
LaBerge, D. (1983). Spatial extent of attention to letters and words. Journal of Experimental Psychology: Human Perception and Performance, 9, 371–379.
LaBerge, D., Brown, V. (1986). Variations in size of the visual field in which targets are presented: An attentional range effect. Perception & Psychophysics, 40(3), 188–200.
Lambert, A., Hockey, R. (1991). Peripheral visual changes and spatial attention. Acta Psychologica, 76, 149–163.
Lavie, N. (1995). Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance, 21, 451–468.
Lehmann, E. L., Stein, C. (1949). On the theory of some non-parametric hypotheses. The Annals of Mathematical Statistics, 20(1), 28–45.
Liu, T., Pestilli, F., Carrasco, M. (2005). Transient attention enhances perceptual performance and fMRI response in human visual cortex. Neuron, 45, 469–477.
Lu, Z.-L., & Dosher, B. (2005). Neurobiology of attention. In L. Itti, G. Rees, & J. Tsotsos (Eds.), (pp. 448–453). New York: Academic Press.
Lu, Z.-L., Dosher, B. A. (1998). External noise distinguishes attention mechanisms. Vision Research, 38, 1183–1198.
Luck, S. J., Thomas, S. J. (1999). What variety of attention is automatically captured by peripheral cues? Perception and Psychophysics, 61(7), 1424–1435.
Macmillan, N. A., & Creelman, C. D. (1991). Detection theory: A user’s guide. Cambridge: Cambridge University Press.
Marcum, J. I. (1947). A statistical theory of target detection by pulsed radar (Research Memorandum RM- 754) (Technical Report). Project RAND, Canada: Douglas Aircraft Company, Inc.
Mueller, S. T., Weidemann, C. T. (2008). Decision noise: An explanation for observed violations of signal detection theory. Psychonomic Bulletin & Review, 15(3), 465–494.
Müller, H. J., Findley, J. M. (1987). Sensitivity and criterion effects in the spatial cuing of visual attention. Perception & Psychophysics, 42, 383–399.
Müller, H. J., Rabbit, P. M. A. (1989). Reflexive and Voluntary Orienting of Visual Attention: Time Course of Activation and Resistance to Interruption. Journal of Experimental Psychology: Human Perception and Performance, 15(2), 315–330.
Müller, N. G., Mollenhauer, M., Rösler, A., Kleinschmidt, A. (2005). The attentional field has a mexican hat distribution. Vision Research, 45, 1129–1137.
Müller-Plath, G. (2008). Localizing subprocesses of visual search by correlating local brain activation in fMRI with response time model parameters. Journal of Neuroscience Methods, 171(2), 316–330.
Nagy, A. L., Sanchez, R. R., Hughes, T. C. (1990). Visual search for color differences with foveal and peripheral vision. Journal of the Optical Society of America A, 7(10), 1995–2001.
Neumann, O. (1987). Beyond capacity: A functional view of attention. In H. Heuer & A. F. Sanders (Eds.), Perspectives on Perception and Action. Hillsdale, NJ: Erlbaum.
Ossandón, J. P., Onat, S., Cazzoli, D., Nyffeler, T., Müri, R., König, P. (2012). Unmasking the contribution of low-level features to the guidance of attention. Neuropsychologia, 50, 3478–3487.
Pestilli, F., Carrasco, M. (2005). Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Research, 45, 1867–1875.
Posner, M. I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32, 3–25.
Posner, M. I., Cohen, Y. (1984). Components of visual orienting. In H. Bouma & D. G. Bouwhuis (Eds.), Attention and Performance X. Hove, UK: Erlbaum.
Prinzmetal, W., H., A., Allen, K., & Edwards, T. (1998). Phenomenology of attention: 1. color, location, orientation, and spatial frequency. Journal of Experimental Psychology: Human Perception and Performance, 24, 261-282.
Prinzmetal, W., Long, V., Leonhardt, J. (2008). Involuntary attention and brightness contrast. Perception & Psychophysics, 70(7), 1139–1150.
Prinzmetal, W., McCool, C., Park, S. (2005). Attention: reaction time and accuracy reveal different mechanisms. Journal of Experimental Psychology: General, 134, 73–92.
Prinzmetal, W., Zvinyatskovskiy, A., Gutierrez, P., Dilem, L. (2009). Voluntary and involuntary attention have different consequences: The effect of perceptual difficulty. The Quarterly Journal of Experimental Psychology, 62, 352–369.
Reynolds, J. H., Pasternak, T., Desimone, R. (2000). Attention increases sensitivity of V4 neurons. Neuron, 26, 703–714.
Ronconi, L., Basso, D., Gori, S., & Facoetti, A. (2013a). TMS on right frontal eye fields induces an inflexible focus of attention. Cerebral Cortex. doi:10.1093/cercor/bhs319
Ronconi, L., Gori, S., Ruffino, M., Molteni, M., & Facoetti, A. (2013b). Zoom-out attentional impairment in children with autism spectrum disorder. Cortex. doi:10.1016/j.cortex.2012.03.005
Schneider, K. A. (2006). Does attention alter appearance? Perception & Psychophysics, 68, 800–814.
Schneider, K. A., Komlos, M. (2008). Attention biases decisions but does not alter appearance. Journal of Vision, 8(15), 1–10.
Schreij, D., Owens, C., Theeuwes, J. (2008). Abrupt onsets capture attention independent of top-down control settings. Perception & Psychophysics, 70(2), 208–218.
Shiu, L., Pashler, H. (1994). Negligible effect of spatial precuing on identification of single digits. Journal of Experimental Psychology: Human Perception and Performance, 20, 1037–1054.
Styles, E. A. (2006). The Psychology of Attention. Oxford: Psychology Press.
Swets, J. A. (1996). Signal Detection Theory and ROC Analysis in Psychology and Diagnostics. Mahwah, NJ: Erlbaum.
Tassinari, G., Aglioti, S., Chelazzi, L., Peru, A., Berlucchi, G. (1994). Do peripheral non-informative cues induce early facilitation of target detection? Vision Research, 34(2), 197–189.
Tassinari, G., Berlucchi, G. (1993). Sensory and attentional components of slowing of manual reaction time to non-fixated visual targets by ipsilateral primes. Vision Research, 33(11), 1525–1534.
Theeuwes, J. (1991). Cross-dimensional perceptual selectivity. Perception & Psychophysics, 50, 184–193.
Treisman, A. M. (1998). Feature binding, attention and object perception. Philosophical Transactions of the Royal Society of London: B, 353, 1295–1306.
Treisman, A. M., Gelade, G. (1980). A feature-integration theory of attention. Cognitive Psychology, 12, 97–136.
Tsushima, Y., Sasaki, Y., Watanabe, T. (2006). Greater disruption due to failure of inhibitory control on an ambiguous distractor. Science, 314(5806), 1786–1788.
Wei, P., Müller, H. J., Pollmann, S., Zhou, X. (2011). Neural correlates of binding features within-or crossdimensions in visual conjunction search: An fMRI study. Neuroimage, 57, 235–241.
Wickens, T. D. (2002). Elementary signal detection theory. Oxford: Oxford University Press.
Wienrich, C., Janczyk, M. (2011) Attention, Perception & Psychophysics, 73, 2044–2052.
Wolfe, J. M. (1994). Guided Search 2.0: A revised model of visual search. Psychonomic Bulletin & Review, 1, 202–238.
Wundt, W. (1880). Grundzüge der physiologischen Psychologie [Principles of Physiological psychology] (2nd ed., Vol. 2). Leipzig: Engelmann.
Yantis, S., Jonides, J. (1984). Abrupt visual onsets and selective attention: evidence from visual search. Journal of Experimental Psychology: Human Perception and Performance, 10, 601–621.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Behavioral measures of accuracy and bias
Let us start from an experimental 3 × 2-design for one subject with the factors ‘cue’ C with the three levels C n , C v , C i (no, valid, or invalid cue) and ‘target’ S with the two levels S 0, S 1 (target absent or present). The dependent measure is the binary ‘response’ R with the values R 0, R 1 (no, yes). If each of the six conditions is presented n times to a subject, a joint frequency distribution as displayed in Table 11 is the result. Since a cue is neither valid nor invalid when there is no target, the conditions C v S 0 and C i S 0 are phenomenologically identical and the respective frequencies are summed.
Accordingly, when computing hit and false alarm rates, the latter were pooled across all cued trials. Equation 1 shows the resulting three hit rates pH n , pH v , pH i and two false alarm rates pF n , pF vi for this situation.
Target detection accuracy pC and response bias pR with pooled false alarms
For assessing target detection accuracy and response bias, the rate of correct responses pC and the rate of yes-responses pR were analyzed for each of the three cueing conditions. For the no-cue condition, they are defined from Table 11 as
and
However, when proceeding in this manner for validly and invalidly cued trials, the six variables pC j and pR j for j = n, v, i would be multicollinear because they were linearly combined from the five variables pH n , pH v , pH i , pF n , pF vi . (For example, the differences pC v − pC i and pR v − pR i were identical then.)
Therefore, the following variables were computed instead:
with the pooled hit rate pH vi = .5 (pH v + pH i ).
Defining pC and pR as linear combinations of pH and pF is statistically useful for the following reasons:
-
1.
At the descriptive level, i.e., in a sample of N subjects, each of the above measures can be regarded as a vector of length N. In terms of the linear model, the five primary measures build up a (N × 5) design matrix
$${\mathbf{X}}\,=\,(\,\overrightarrow{pH}_{n}, \overrightarrow{pH}_{\rm v}, \overrightarrow{pH}_{{\rm i}}, \overrightarrow{pF}_{n}, \overrightarrow{pF}_{{\rm vi}}),$$as well as the five secondary measures
$${\mathbf{Y}}\,=\,(\,\overrightarrow{pC}_n, \overrightarrow{pC}_{\rm v}, \overrightarrow{pC}_{\rm i}, \overrightarrow{pR}_n, \overrightarrow{pR}_{{\rm vi}}\,).$$It is easily shown with the help of basic matrix algebra that the columns of Y are linearly independent if and only if the columns of X are linearly independent.
Proof
Consider the (5 × 5) transformation matrix
and the (N × 5) translation matrix
Then, the design matrix Y results from the design matrix X by the affine linear transformation
The proposition follows from A having full rank (det A = .0625).
-
2.
At the theoretical level, let
$${\mathbf{x}}\,=\,(\,\pi(H)_n, \pi(H)_{\rm v}, \pi(H)_{\rm i}, \pi(F)_n, \pi(F)_{{\rm vi}}\,)^{\prime}$$and
$${\mathbf{y}}\,=\,(\,\pi(C)_n, \pi(C)_{\rm v}, \pi(C)_{\rm i}, \pi(R)_n, \pi(R)_{{\rm vi}}\,)^{\prime}$$denote the two 5-dimensional random vectors that produce design matrices as the above X and Y when realized N times independently. With the above (5 × 5)-matrix A and the vector
$${\mathbf{b}}\,=\, (.5 , .5, .5, 0, 0)^{\prime}\,,$$the random vector y results from the random vector x by the linear transformation
$${\mathbf{y}}\,=\,{\mathbf{A}}\, {\mathbf{x}} + {\mathbf{b}},$$and for their means and covariance matrices the relations
$$\begin{aligned} E({\mathbf{y}})&= {\mathbf{A}}\, E({\mathbf{x}}) + {\mathbf{b}},\\ V({\mathbf{y}})&= {\mathbf{A}}\, V({\mathbf{x}})\, {\mathbf{A}}^{\prime} \end{aligned}$$hold. Furthermore, since A is symmetric with full rank, x is multivariately normally distributed if and only if y is is multivariately normally distributed. Therefore, it makes no difference for any common inference statistic whether it is carried out on the set of primary measures as defined in Eq. (1) or the set of secondary measures as defined in Eqs. (2)–(6).
To summarize, it is statistically equivalent whether the hits and false alarms pH and pF are analyzed or the target detection accuracy and response bias pC and pR. However, the latter is more convenient for interpretation.
Appendix 2
Variants of signal detection theory (SDT)
The following theoretical section is divided into three subsections: First, we will briefly recapitulate the standard SDT model and its parameters. Then, we will discuss how it can be extended to our five stimulus classes. Third, we will generalize the model by weakening some assumptions. The parameter estimations from the best fitting model variant were used for drawing conclusions on mechanisms involved in the cueing effects.
Although most readers will be familiar with the standard model, we will recapitulate it briefly as a starting point for the modifications introduced below. In standard SDT (Marcum, 1947; psychophysical adaptation by Green & Swets, 1966), the sensory representations evoked by targets (S 1) and non-targets (S 0) are assumed to range along a one-dimensional random variable which is normally distributed for targets and nontargets with equal variances but different means (see Fig. 6). The sensitivity d′ is conceptualized as the difference between the target and nontarget mean in units of standard deviation. An alternative sensitivity measure is A z , the area under the ROC curve (isosensitivity curve for a given d′ value), which can be interpreted as the probability that the sensory representation of the target exceeds that of the non-target when both are independently sampled from the two conditional distributions. A z is identical to \(F(d^{\prime}/\sqrt{2})\), with F denoting the cumulative distribution function of the standard normal. The raw criterion measure λ is a fixed variable value above which the observer responds “yes”. For interpreting it as bias, the criterion measure is usually centered at the point of equal hit and miss rate ζ (which can sensibly be interpreted as point of zero bias and which equals d′/2 when the variances are equal). The bias is thus defined as c = λ − d′/2 (Macmillan & Creelman, 1991; also termed λcenter in Wickens, 2002). An alternative measure of bias is the so-called β (with or without logarithm), the ratio of the two conditional probability densities at the criterion value c. Note that log(β) is identical to c·d′ (Wickens, p. 30).
SDT for five stimulus classes
There exist several attempts to apply SDT to spatial cueing experiments (e.g., Bashinsky & Bacharach, 1980; Müller & Findley, 1987; Prinzmetal et al., 2008). Mostly, the three trial types (no cue, valid cue, invalid cue) were treated as three different pairs of nontarget and target distributions, yielding three independent estimations of the sensitivity and the criterion/bias measure.
In the present experiments, however, there were five stimulus classes (nontarget trials without and with cue, and target trials without, with valid, or with invalid cue). SDT can thus be applied as usual to the uncued trials, assuming a pair of nontarget and target distribution and estimating the sensitivity d ′ n , and the criterion/bias λ n /c n . However, since the criterion is estimated solely from the false alarm rate of which there is only one in the sample of all cued trials, the remaining three stimulus classes (target with valid cue, target with invalid cue, nontarget with cue) have to be treated together in one model.
How many parameters does this latter situation—the SDT with three stimulus classes—contain? First, the two target distributions (validly cued and invalidly cued targets) with their two means μ v and μ i constitute two sensitivies d′(v) and d′(i). Second, and now the situation requires second thought, the nontarget distribution with its false alarm rate constitutes one criterion, which will be indexed λ(vi) in the following. The bias c is the difference between the criterion value, of which there is one, and the point of equal false alarm and miss rate (‘zero bias’), of which there are two. Obviously, it does not make much sense to compute two different biases from only one criterion. (Moreover, the difference between these two biases would directly reflect the difference between the two sensitivities.) It seems more sensible to redefine the point of ‘zero bias’ instead: If there are two target types, validly and invalidly cued ones, we defined an observer to have ‘zero bias’ if his/her criterion met the point at which the false alarm rate matches the pooled miss rate across the two target types. Consequently, we defined the bias as the difference between the actual criterion and the point of zero bias. To estimate its value, we proceeded from the already estimated parameters λ(vi), d′(v), and d′(v), pooled the estimated miss rates, identified the point ζ at which the pooled miss rate equaled the estimated false alarm rate, and then computed the bias as c(vi): = λ(vi) − ζ.
The resulting graphics in the upper panels of Fig. 4 may illustrate the procedure. (The reader should not be confused by the three criteria λC, λU, λL and accordingly three zero bias and three bias values, which correspond to the three payoff conditions that were realized in the experiments but disregarded in the above explanation for didactic reasons. The subscript c a instead of c refers to the unequal variance model, which will be explained in the next subsection.)
Generalizations of model assumptions
To judge whether the distributional assumptions of the model were appropriate for the data, three payoff conditions had been realized in experiment 1 and 2, and two in experiment 3 (see “Methods” sections of the experiments). From the data of every subject, we obtained maximum-likelihood estimations of the mean of the target distribution in the uncued case and of the two means of the two target distributions in the cued case, with three instead of one criterion λ each in experiment 1 and 2, and two times two criteria in experiment 3. The nontarget distributions were always standardized to a mean of 0 and a variance of 1. This was done with and without the constraint of equal variances. Then, for every experiment and every subject, a goodness-of-fit test was computed with the help of the χ2 statistic. At a significance level of α = .05, the Gaussian model with equal variances was rejected for five of the six subjects in experiment 1, three of the six subjects in experiment 2, and five of the six subjects in experiment 3. However, when allowing for heterogenous variances across all target distributions, the Gaussian model remained statistically unrefuted at α = 0.05 for every subject in every experiment. Further, generalizations like giving up the normal distribution (Green & Swets, 1966) or introducing decision noise see, e.g.,(see, e.g., Bonnel & Miller, 1994; Mueller & Weidemann, 2008; Benjamin, Diaz, & Wee, 2009) did not further improve the fit. We thus interpreted the parameters from the unequal variance Gaussian SDT model.
Several ideas exist on how to generalize d′ when allowing for unequal variances (Wickens, 2002). We used d a , the difference between the mean of the target and the nontarget distribution in units of pooled standard deviation (which reduces to d′ if variances are equal). Since d a estimations depend critically on the variances being accurately estimated, we also analyzed A z , the area under the ROC curve, the estimation of which is more robust against changes in the underlying distributions or parameters and even nonnormality.
For investigating bias shifts in the heterogeneous variance model, we first looked at the raw criterion values λcon, λbal, λlib for the three payoff conditions. Then, in loose analogy to the centered criterion c in standard SDT, we standardized the raw criteria in each pair of target-nontarget distributions by subtracting the point of zero bias \(\zeta\) and dividing by the pooled standard deviation, resulting in so-called c a -values (Wickens, 2002). (We refrained from using the bias measures β or log(β) for assessing criterion shifts because the maximum of a probability density function varies with its width, and consequently the ratios of density values are not comparable between pairs of distributions with different variance ratios, e.g., different subjects or cueing conditions.)
Note that the here described standardization of d′ and c into d a and c a , accounting for the unequal variance situation, does not interfere with the above considerations on how many sensitivities and biases can sensibly be estimated for the cued and the uncued case, accounting for the five stimulus classes.
In total, each subject came with three sensitivity estimates d a (n), d a (v), and d a (i) (for uncued, validly cued and invalidly cued targets), and two bias estimates c a (n) and c a (vi) (for uncued and cued nontargets). The situation thereby mirrored the empirical situation in which three target detection accuracies pC and two response biases pR were computed. A difference existed only with regard to the three payoff conditions, which in the empirical situation triplicated both the number of accuracy measures pC and bias measures pR but in the SDT situation only the bias estimates.
Rights and permissions
About this article
Cite this article
Müller-Plath, G., Klöckner, N. Exogenous attention can be counter-selective: onset cues disrupt sensitivity to color changes. Psychological Research 78, 222–247 (2014). https://doi.org/10.1007/s00426-013-0489-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-013-0489-5