Abstract
Main conclusion
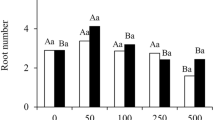
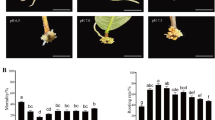
Optimal levels of indole-3-butyric acid (IBA) applied at the stem base promote adventitious root (AR) initiation and primordia formation, thus promoting the rooting of leafy micro-cuttings of tetraploid Robinia pseudoacacia.
Abstract
Tetraploid Robinia pseudoacacia L. is a widely cultivated tree in most regions of China that has a hard-rooting capability, propagated by stem cuttings. This study utilizes histological, physiological, and transcriptomic approaches to explore how root primordia are induced after indole butyric acid (IBA) treatment of micro-cuttings. IBA application promoted cell divisions in some cells within the vasculature, showing subcellular features associated with adventitious root (AR) founder cells. The anatomical structure explicitly showed that AR initiated from the cambium layer and instigate the inducible development of AR primordia. Meanwhile, the hormone data showed that similar to that of indole-3-acetic acid, the contents of trans-zeatin and abscisic acid peaked at early stages of AR formation and increased gradually in primordia formation across the subsequent stages, suggesting their indispensable roles in AR induction. On the contrary, 24-epibrassinolide roughly maintained at extremely high levels during primordium initiation thoroughly, indicating its presence was involved in cell-specific reorganization during AR development. Furthermore, antioxidant activities transiently increased in the basal region of micro-cuttings and may serve as biochemical indicators for distinct rooting phases, potentially aiding in AR formation. Transcriptomic analysis during the early stages of root formation shows significant downregulation of the abscisic acid and jasmonate signaling pathways, while ethylene and cytokinin signaling seems upregulated. Network analysis of genes involved in carbon metabolism and photosynthesis indicates that the basal region of the micro-cuttings undergoes rapid reprogramming, which results in the breakdown of sugars into pyruvate. This pyruvate is then utilized to fuel the tricarboxylic acid cycle, thereby sustaining growth through aerobic respiration. Collectively, our findings provide a time-course morphophysiological dissection and also suggest the regulatory role of a conserved auxin module in AR development in these species.











Similar content being viewed by others
Data availability
Raw sequence files and read count files are publicly available in the NCBI’s BioProject repository. Gene functional annotation is available in the supplementary material of this article. All other data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AR:
-
Adventitious root
- TRP:
-
Tetraploid Robinia pseudoacacia
- TEM:
-
Transmission electron microscopy
- ROS:
-
Reactive oxygen species
- IBA:
-
Indole-3-butyric acid
- IAA:
-
Indole-3-acetic acid
- ABA:
-
Abscisic acid
- Tz:
-
Trans-Zeatin
- BL:
-
24-Epibrassinolide
- SA:
-
Salicylic acid
- HAE:
-
Hours after excision
- DEGs:
-
Differentially expressed genes
References
Agarwal S, Sairam R, Srivastava G, Tyagi A, Meena R (2005) Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci 169(3):559–570
Agulló-Antón MÁ, Sánchez-Bravo J, Acosta M, Druege U (2011) Auxins or sugars: what makes the difference in the adventitious rooting of stored carnation cuttings? J Plant Growth Regul 30(1):100–113
Agulló-Antón MÁ, Ferrández-Ayela A, Fernández-García N, Nicolás C, Albacete A, Pérez-Alfocea F, Sánchez-Bravo J, Pérez-Pérez JM, Acosta M (2014) Early steps of adventitious rooting: morphology, hormonal profiling and carbohydrate turnover in carnation stem cuttings. Physiol Plant 150(3):446–462
Ahkami AH, Lischewski S, Haensch KT, Porfirova S, Hofmann J, Rolletschek H, Melzer M, Franken P, Hause B, Druege U (2009) Molecular physiology of adventitious root formation in Petunia hybrida cuttings: involvement of wound response and primary metabolism. New Phytol 181(3):613–625
Atkinson JA, Rasmussen A, Traini R, Voß U, Sturrock C, Mooney SJ, Wells DM, Bennett MJ (2014) Branching out in roots: uncovering form, function, and regulation. Plant Physiol 166(2):538–550
Baque MA, Hahn E-J, Paek K-Y (2010) Induction mechanism of adventitious root from leaf explants of Morinda citrifolia as affected by auxin and light quality. In Vitro Cellular & Developmental Biology-Plant 46:71–80
Batish DR, Singh HP, Kaur S, Kohli RK, Yadav SS (2008) Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J Plant Physiol 165(3):297–305
Bellini C, Pacurar DI, Perrone I (2014) Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol 65:639–666
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13(8):1194–1202
Chini A, Fonseca S, Fernandez G, Adie B, Chico J, Lorenzo O, García-Casado G, López-Vidriero I, Lozano F, Ponce M (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448(7154):666–671
Da Costa CT, De Almeida MR, Ruedell CM, Schwambach J, Maraschin FDS, Fett-Neto AG (2013) When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci 4:133
Dawood T, Yang X, Visser EJ, Te Beek TA, Kensche PR, Cristescu SM, Lee S, Floková K, Nguyen D, Mariani C (2016) A co-opted hormonal cascade activates dormant adventitious root primordia upon flooding in Solanum dulcamara. Plant Physiol 170(4):2351–2364
Dewitte W, Scofield S, Alcasabas AA, Maughan SC, Menges M, Braun N, Collins C, Nieuwland J, Prinsen E, Sundaresan V (2007) Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc Natl Acad Sci 104(36):14537–14542
Dong S, Liu M, Dai F, Wu Y, Shan S, Ding R (2013) Variation of endogenous hormone contents in softwood cuttings of Armeniaca sibirica during adventitious root formation. Non-Wood for Res 31:108–114
Druege U, Hilo A, Pérez-Pérez JM, Klopotek Y, Acosta M, Shahinnia F, Zerche S, Franken P, Hajirezaei MR (2019) Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation. Ann Bot 123(6):929–949
Ge SX, Son EW, Yao R (2018) iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics 19(1):1–24
Gibson SI (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8(1):93–102
Guan L, Murphy AS, Peer WA, Gan L, Li Y, Cheng Z-M (2015) Physiological and molecular regulation of adventitious root formation. Crit Rev Plant Sci 34(5):506–521
Gutierrez L, Bussell JD, Pacurar DI, Schwambach J, Pacurar M, Bellini C (2009a) Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21(10):3119–3132
Gutierrez L, Bussell JD, Păcurar DI, Schwambach J, Păcurar M, Bellini C (2009b) Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21(10):3119–3132
Gutierrez L, Mongelard G, Floková K, Păcurar DI, Novák O, Staswick P, Kowalczyk M, Păcurar M, Demailly H, Geiss G (2012) Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 24(6):2515–2527
Hagen G (2015) Auxin Signal Transduction. Essays Biochem 58:1–12
Ibrahim W, Qiu CW, Zhang C, Cao F, Shuijin Z, Wu F (2019) Comparative physiological analysis in the tolerance to salinity and drought individual and combination in two cotton genotypes with contrasting salt tolerance. Physiol Plant 165(2):155–168
Kanehisa M, Sato Y, Morishima K (2016) BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol 428(4):726–731
Klopotek Y, Haensch K-T, Hause B, Hajirezaei M-R, Druege U (2010) Dark exposure of petunia cuttings strongly improves adventitious root formation and enhances carbohydrate availability during rooting in the light. J Plant Physiol 167(7):547–554
Klopotek Y, Franken P, Klaering H-P, Fischer K, Hause B, Hajirezaei M-R, Druege U (2016) A higher sink competitiveness of the rooting zone and invertases are involved in dark stimulation of adventitious root formation in Petunia hybrida cuttings. Plant Sci 243:10–22
Lakehal A, Bellini C (2019) Control of adventitious root formation: insights into synergistic and antagonistic hormonal interactions. Physiol Plant 165(1):90–100
Lakehal A, Chaabouni S, Cavel E, Le Hir R, Ranjan A, Raneshan Z, Novák O, Păcurar DI, Perrone I, Jobert F (2019) A molecular framework for the control of adventitious rooting by TIR1/AFB2-Aux/IAA-dependent auxin signaling in Arabidopsis. Mol Plant 12(11):1499–1514
Larriba E, Sánchez-García AB, Martínez-Andújar C, Albacete A, Pérez-Pérez JM (2021) Tissue-specific metabolic reprogramming during wound-induced organ formation in tomato hypocotyl explants. Int J Mol Sci 22(18):10112
Larriba E, Nicolás-Albujer M, Sánchez-García AB, Pérez-Pérez JM (2022) Identification of transcriptional networks involved in De Novo organ formation in tomato hypocotyl explants. Int J Mol Sci 23(24):16112
Lechner M, Findeiß S, Steiner L, Marz M, Stadler PF, Prohaska SJ (2011) Proteinortho: detection of (co-) orthologs in large-scale analysis. BMC Bioinformatics 12(1):1–9
Li S-W, Xue L, Xu S, Feng H, An L (2009) IBA-induced changes in antioxidant enzymes during adventitious rooting in mung bean seedlings: the role of H2O2. Environ Exp Bot 66(3):442–450
Li S-W, Shi R-F, Leng Y, Zhou Y (2016) Transcriptomic analysis reveals the gene expression profile that specifically responds to IBA during adventitious rooting in mung bean seedlings. BMC Genomics 17(1):1–23
Li F, Sun C, Li X, Yu X, Luo C, Shen Y, Qu S (2018a) The effect of graphene oxide on adventitious root formation and growth in apple. Plant Physiol Biochem 129:122–129
Li S-W, Zeng X-Y, Leng Y, Feng L, Kang X-H (2018b) Indole-3-butyric acid mediates antioxidative defense systems to promote adventitious rooting in mung bean seedlings under cadmium and drought stresses. Ecotoxicol Environ Saf 161:332–341
Li K, Tian H, Tahir MM, Li S, Chen S, Fan L, Liu Z, Mao J, Zhang D (2022) Transcriptome analysis reveals that cytokinins inhibit adventitious root formation through the MdRR12-MdCRF8 module in apple rootstock. Plant Sci 318:111220
Lu N, Dai L, Luo Z, Wang S, Wen Y, Duan H, Hou R, Sun Y, Li Y (2017) Characterization of the transcriptome and gene expression of tetraploid black locust cuttings in response to etiolation. Genes 8(12):345
Ma W, Zhang S, Wang J, Sun X, Zhao H, Ning Y (2013) Endogenous hormones, nutritive material and phenolic acid variation in cuttings of Japanese larch during rooting. Acta Botan Boreali-Occiden Sin 33(1):109–115
Mauriat M, Petterle A, Bellini C, Moritz T (2014) Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J 78(3):372–384
Min L, Li Y, Hu Q, Zhu L, Gao W, Wu Y, Ding Y, Liu S, Yang X, Zhang X (2014) Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol 164(3):1293–1308
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628
Munir MZ, Ud Din S, Imran M, Zhang Z, Pervaiz T, Han C, Un Nisa Z, Bakhsh A, Atif Muneer M, Sun Y (2021) Transcriptomic and Anatomic Profiling Reveal Etiolation Promotes Adventitious Rooting by Exogenous Application of 1-Naphthalene Acetic Acid in Robinia pseudoacacia L. Forests 12(6):789
Nag S, Saha K, Choudhuri M (2001) Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J Plant Growth Regul 20(2):182–194
Niu Q, Zong Y, Qian M, Yang F, Teng Y (2014) Simultaneous quantitative determination of major plant hormones in pear flowers and fruit by UPLC/ESI-MS/MS. Anal Methods 6(6):1766–1773
Pacurar DI, Perrone I, Bellini C (2014) Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol Plant 151(1):83–96
Pan X, Yang Z, Xu L (2021) Dual roles of jasmonate in adventitious rooting. J Exp Bot 72(20):6808–6810
Pasternak T, Groot EP, Kazantsev FV, Teale W, Omelyanchuk N, Kovrizhnykh V, Palme K, Mironova VV (2019) Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol 180(3):1725–1739
Péret B, Swarup K, Ferguson A, Seth M, Yang Y, Dhondt S, James N, Casimiro I, Perry P, Syed A (2012) AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell 24(7):2874–2885
Pitaksaringkarn W, Matsuoka K, Asahina M, Miura K, Sage-Ono K, Ono M, Yokoyama R, Nishitani K, Ishii T, Iwai H (2014) XTH 20 and XTH 19 regulated by ANAC 071 under auxin flow are involved in cell proliferation in incised Arabidopsis inflorescence stems. Plant J 80(4):604–614
Qi H, Cai H, Liu X, Liu S, Ding C, Xu M (2022) The cytokinin type-B response regulator PeRR12 is a negative regulator of adventitious rooting and salt tolerance in poplar. Plant Sci 325:111456
Qingmin W, Weixiu P, Junpei Z, Dong P (2006) Histolog ical and Hormonal Characters during the Rhizogenesis of in Vitro Walnut Shoots. Acta Horticulturae Sinica 33(2):255
Ramírez-Carvajal GA, Morse AM, Dervinis C, Davis JM (2009) The cytokinin type-B response regulator PtRR13 is a negative regulator of adventitious root development in Populus. Plant Physiol 150(2):759–771
Savelli B, Li Q, Webber M, Jemmat AM, Robitaille A, Zamocky M, Mathé C, Dunand C (2019) RedoxiBase: A database for ROS homeostasis regulated proteins. Redox Biol 26:101247
Steffens B, Rasmussen A (2016) The physiology of adventitious roots. Plant Physiol 170(2):603–617
Steffens B, Wang J, Sauter M (2006) Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223:604–612
Suárez E, Alfayate C, Pérez-Francés JF, Rodríguez-Pérez JA (2018) Structural and ultrastructural variations in in vitro and ex vitro rooting of microcuttings from two micropropagated Leucospermum (Proteaceae). Sci Hortic 239:300–307
Sun J, Chen W, Ji J, Jiang Z, Shi S (2019) Comparison of physiological and anatomical characteristics between seedlings and graftings derived from old Platycladus orientalis. Scientia Silvae Sinicae 55(9):41–49
Syros T, Yupsanis T, Zafiriadis H, Economou A (2004) Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J Plant Physiol 161(1):69–77
Tran S, Ison M, Ferreira Dias NC, Ortega MA, Chen Y-FS, Peper A, Hu L, Xu D, Mozaffari K, Severns PM (2023) Endogenous salicylic acid suppresses de novo root regeneration from leaf explants. PLoS Genet 19(3):e1010636
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28(5):511–515
Vidal N, Arellano G, San-José MC, Vieitez A, Ballester A (2003) Developmental stages during the rooting of in-vitro-cultured Quercus robur shoots from material of juvenile and mature origin. Tree Physiol 23(18):1247–1254
Villacorta-Martín C, Sánchez-García AB, Villanova J, Cano A, van de Rhee M, de Haan J, Acosta M, Passarinho P, Pérez-Pérez JM (2015) Gene expression profiling during adventitious root formation in carnation stem cuttings. BMC Genomics 16:1–18
Wang X, Zhao Z, Quan J (2011) Indole-3-butyric acid on rooting and endogenous plant hormones in tetraploid and diploid Robinia pseudoacacia hardwood cuttings. Phyton-Revista Internacional De Botanica Experimental 23:93–99
Wang Z, Hua J, Yin Y, Gu C, Yu C, Shi Q, Guo J, Xuan L, Yu F (2019) An integrated transcriptome and proteome analysis reveals putative regulators of adventitious root formation in Taxodium ‘Zhongshanshan.’ Int J Mol Sci 20(5):1225
Wang Q, Zhang J, Zhong C, Zhang Y, Wei Y, Meng J (2020) Variation of endogenesis hormone and nutritive matter concentration in Chukrasia tabularis cuttings during rooting. J Cent South Univ for Technol 40:111–119
Wang Y, Sun L, Wang R, Li H, Zhu Z (2023a) The AP2 transcription factors TOE1/TOE2 convey Arabidopsis age information to ethylene signaling in plant de novo root regeneration. Planta 257(1):1
Wang Z, Zhang X, Lei W, Zhu H, Wu S, Liu B, Ru D (2023b) Chromosome-level genome assembly and population genomics of Robinia pseudoacacia reveal the genetic basis for its wide cultivation. Communications Biology 6(1):797
Wei K, Ruan L, Wang L, Cheng H (2019) Auxin-induced adventitious root formation in nodal cuttings of Camellia sinensis. Int J Mol Sci 20(19):4817
Xiao-ling W, Zhong Z, Jin-e Q (2011) Effects of planting time on endogenous hormones and oxidase in tetraploid Robinia pseudoacacia softwood cuttings. J Beijing Forestry Univ 33(6):102–106
Xu X, Chen M, Ji J, Xu Q, Qi X, Chen X (2017) Comparative RNA-seq based transcriptome profiling of waterlogging response in cucumber hypocotyls reveals novel insights into the de novo adventitious root primordia initiation. BMC Plant Biol 17(1):1–13
Xu P, Fang S, Chen H, Cai W (2020) The brassinosteroid-responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J 104(1):59–75
XU X, (2009) Changes of soluble proteins and content of endohormone in the process of striking roots of Malus prunifolia borkh. J Nanjing Forestry Univ 52(02):60
Zeng Y, Verstraeten I, Trinh HK, Heugebaert T, Stevens CV, Garcia-Maquilon I, Rodriguez PL, Vanneste S, Geelen D (2021) Arabidopsis hypocotyl adventitious root formation is suppressed by ABA signaling. Genes 12(8):1141
Zhang J, Liu Y, Wang F, Ma J, Wei L, Jiang S (2018) Physiological and biochemical characteristics of Tamarix taklamakanensis cuttings during rooting stages. Acta Botan Boreali-Occiden Sin 38(3):484–492
Zhang G, Zhao F, Chen L, Pan Y, Sun L, Bao N, Zhang T, Cui C-X, Qiu Z, Zhang Y (2019a) Jasmonate-mediated wound signalling promotes plant regeneration. Nature Plants 5(5):491–497
Zhang Y, Zhan C, Liu M, Xia W, Wang N (2019b) Comprehensive analysis of dynamic gene expression and investigation of the roles of hydrogen peroxide during adventitious rooting in poplar. BMC Plant Biol 19(1):1–16
Dawood T, Rieu I, Wolters-Arts M, Derksen EB, Mariani C, Visser EJ (2014) Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB Plants 6:plt058
Delfin JC, Kanno Y, Seo M, Kitaoka N, Matsuura H, Tohge T, Shimizu T (2022) AtGH3. 10 is another jasmonic acid‐amido synthetase in Arabidopsis thaliana. The Plant Journal 110 (4):1082–1096
Dob A, Lakehal A, Novak O, Bellini C (2021) Jasmonate inhibits adventitious root initiation through repression of CKX1 and activation of RAP2. 6L transcription factor in Arabidopsis. J Exp Bot 72 (20):7107–7118
Geiss G, Gutierrez L, Bellini C (2018) Adventitious root formation: new insights and perspectives. Ann Plant Rev Online:127–156
Guan L, Li Y, Huang K, Cheng Z-MM (2020) Auxin regulation and MdPIN expression during adventitious root initiation in apple cuttings. Horticult Res 7
Li M, Leung DW (2000) Starch Accumulation Is Associated with Adventitious RootFormation in Hypocotyl Cuttings of Pinus radiata. J Plant Growth Regulation 19 (4)
Li H, Yao L, Sun L, Zhu Z (2020) ETHYLENE INSENSITIVE 3 suppresses plant de novo root regeneration from leaf explants and mediates age-regulated regeneration decline. Development 147 (9):dev179457
Su L-J, Zhang J-H, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng Z-Y (2019) Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative medicine and cellular longevity 2019
Zaman M, Kurepin LV, Catto W, Pharis RP (2016) Evaluating the use of plant hormones and biostimulators in forage pastures to enhance shoot dry biomass production by perennial ryegrass (Lolium perenne L.). J Sci Food Agricult 96 (3):715–726
Zhao P, Zhang J, Chen S, Zhang Z, Wan G, Mao J, Wang Z, Tan S, Xiang C (2023) ERF1 inhibits lateral root emergence by promoting local auxin accumulation and repressing ARF7 expression. Cell Rep 42 (6)
Funding
This research was funded by the National Nature Science Foundation of China, grant number 31971675, and the National Key R&D Program of China, grant number 2017YFD0600503. Research in JMP-P laboratory was funded by the Ministerio de Ciencia e Innovación of Spain, grant numbers BIO2015-64255-R and RTI2018-096505-B-I00.
Author information
Authors and Affiliations
Contributions
Conceptualization, YL, JMP-P, and SU planned and carried out the experiments and prepared the first draft of the article. EL and SU prepared results and statistical analysis. MZM, YL, YS, SG, TP, UM, EL, JMP-P, and ZM participated in the design of the study and prepared the final version of the manuscript visualization. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Additional information
Communicated by Stefan de Folter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uddin, S., Munir, M.Z., Larriba, E. et al. Temporal profiling of physiological, histological, and transcriptomic dissection during auxin-induced adventitious root formation in tetraploid Robinia pseudoacacia micro-cuttings. Planta 259, 66 (2024). https://doi.org/10.1007/s00425-024-04341-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-024-04341-1