Abstract
Main conclusion
The biostimulant Hanseniaspora opuntiae regulates Arabidopsis thaliana root development and resistance to Botrytis cinerea.
Abstract
Beneficial microbes can increase plant nutrient accessibility and uptake, promote abiotic stress tolerance, and enhance disease resistance, while pathogenic microorganisms cause plant disease, affecting cellular homeostasis and leading to cell death in the most critical cases. Commonly, plants use specialized pattern recognition receptors to perceive beneficial or pathogen microorganisms. Although bacteria have been the most studied plant-associated beneficial microbes, the analysis of yeasts is receiving less attention. This study assessed the role of Hanseniaspora opuntiae, a fermentative yeast isolated from cacao musts, during Arabidopsis thaliana growth, development, and defense response to fungal pathogens. We evaluated the A. thaliana–H. opuntiae interaction using direct and indirect in vitro systems. Arabidopsis growth was significantly increased seven days post-inoculation with H. opuntiae during indirect interaction. Moreover, we observed that H. opuntiae cells had a strong auxin-like effect in A. thaliana root development during in vitro interaction. We show that 3-methyl-1-butanol and ethanol are the main volatile compounds produced by H. opuntiae. Subsequently, it was determined that A. thaliana plants inoculated with H. opuntiae have a long-lasting and systemic effect against Botrytis cinerea infection, but independently of auxin, ethylene, salicylic acid, or jasmonic acid pathways. Our results demonstrate that H. opuntiae is an important biostimulant that acts by regulating plant development and pathogen resistance through different hormone-related responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During their lifespan, plants interact with multiple microbes, such as bacteria, oomycetes, filamentous fungi, and yeasts. It is well known that microbes can reside in the phyllosphere, endosphere, and rhizosphere regions (Wang et al. 2016; Hawkins and Crawford 2018; Khare et al. 2018; Jones et al. 2019). Some microbes can benefit plant growth and help them survive in changing environments, while others cause many plant diseases (Compant et al. 2010; Dean et al. 2012; Contreras-Cornejo et al. 2014). The most studied beneficial bacteria and filamentous fungi belong to the Bacillus and Trichoderma genera (Wang et al. 2016; Nieto-Jacobo et al. 2017; Jones et al. 2019; Kang et al. 2019), whereas yeasts have received the least attention. On the other hand, plant-associated pathogens include bacterial and fungal agents, such as Erwinia, Pseudomonas, Phytophthora, and Botrytis, among other genera (Mansfield et al. 2012; Dean et al. 2012; De Simone et al. 2020; Donati et al. 2020).
When confronted by microbes, plants use a multifaceted defense system to identify foreign organisms and turn the related signals into defense, such as expressing plant defense response genes. Depending on the microbe lifestyle, the plant immune system employs a wide range of different strategies, which include changes in gene transcription, protein regulation, epigenetic modifications, small RNAs biosynthesis, signal molecules, and accumulation of phytohormones, which can induce defense at a local and/or systemic levels throughout plant organs (Bulgarelli et al. 2013; Mikiciński et al. 2016; Alonso et al. 2019; Fiorilli et al. 2020). At the same time, a balance exists between defense response and growth tradeoff (Cai and Aharoni 2022).
Arabidopsis thaliana is a well-described model organism for plant–microbe interactions analysis (Dubrovsky et al. 1994). This model has allowed the study of host and non-host pathogen interaction systems, root-associated microbiomes, and endophytic microorganisms (Zimmerli et al. 2004; Contreras-Cornejo et al. 2009: Wang et al. 2016; González-Pérez et al. 2018, 2022; Kudjordjie et al. 2021). Thus, the use of A. thaliana has brought to light the basis of plant immunity, such as those mechanisms involved in the perception of microbe-associated molecular patterns (MAMPs) in MAMP-triggered immunity and Resistance (R)-gene function in the signaling of effector-triggered immunity (Jones and Dangl 2006; Li et al. 2020). Although these investigations have centered on bacteria, filamentous fungi, and oomycetes, A. thaliana is also used as a model organism to study plant–yeast interactions (Wang et al. 2016). Knowledge of plant–yeast interactions would be helpful for a more comprehensive understanding of scenarios of plant immunity responses related to beneficial microorganisms.
Members of Hanseniaspora yeast genus are found in grape, citrus, and cacao musts, which are usually the most abundant microorganisms at the beginning of the fermentation (Ferreira-Saab et al. 2018; Hu et al. 2020; Saubin et al. 2020). Hanseniaspora opuntiae was isolated from the Opuntia ficus-indica rot (Cadez et al. 2003) and was considered a biocontrol agent against fungal plant pathogens, such as Corynespora cassiicola and Botrytis cinerea (Ferreira-Saab et al. 2018). Previously, we reported that A. thaliana plants treated with compounds released by the biocontrol yeast H. opuntiae [H. opuntiae-Filtrates (HoFs)] were resistant to the necrotrophic fungus Botrytis cinerea. We observed that HoFs induced the plant defense response in a dose-dependent manner at local and systemic levels. Moreover, by performing an RNA-seq analysis, we identified that HoFs triggered different plant defense response pathways compared to other previously described biocontrol agents (Ferreira-Saab et al. 2018).
In this study, we explored the role of H. opuntiae in A. thaliana growth, development, and defense response to the fungal pathogen B. cinerea. We established that H. opuntiae had a strong auxin-like effect on A. thaliana root development during the direct and indirect in vitro interaction. Afterward, we analyzed the resistance to B. cinerea in plants inoculated with H. opuntiae. Our results suggest that this yeast induces plant resistance to fungal infection by cooperating with multiple hormonal response pathways. We propose that H. opuntiae acts as a biostimulant capable of improving plant growth and controlling fungal pathogens.
Materials and methods
Plant material and growth conditions
Seeds of A. thaliana Col-0 wild type, DR5:uidA auxin reporter line, rhd6 root hair defective mutant and mutants related to auxin- (axr2-1 and tir1), ethylene signaling (ein3), salicylic acid (eds5, npr1-1 and npr3-1), and jasmonic acid (jar1) were used in this study. Seeds were surface sterilized with 70% (v/v) ethanol for five minutes and 96% (v/v) ethanol for five minutes. After ethanol washing, seeds were grown in square Petri dishes on 0.2X Murashige and Skoog (MS) growth medium, pH 5.7, containing 0.5% (w/v) sucrose and 0.8% (w/v) agar (Murashige and Skoog 1962). Seeds were stratified for 2 days at 4 ºC in the dark and then the Petri dishes were incubated in a vertical position at 22 ± 2 ºC in a growth chamber with a 16-h photoperiod (120 μmol m−2 s−1).
Yeast strain and growth conditions
Hanseniaspora opuntiae CCMA 0760 from CCMA (Culture Collection of Agricultural Microbiology, http://www.ccma.dbi.ufla.br/; Universidade Federal de Lavras) was the yeast strain used to perform the experiments. Yeast cells were grown in 0.67% Yeast Nitrogen Base (YNB) supplemented with 2% glucose. Two percent of agar was added to a prepared solid medium. Yeast was grown on YNB plates for 2 days at 28 °C, and the yeast cell suspension was obtained in a YNB liquid medium. The total cell number was calculated in a Neubauer chamber under 40 × magnification in a Zeiss Axioskop 2 microscope using an objective Plan Neofluar 40x/0.75 Ph2.
H. opuntiae–A. thaliana interactions
Two co-culture conditions were evaluated: direct and indirect treatments described in the Results section. The yeast inoculation was carried out seven days after germination of A. thaliana seeds (Col-0, DR5:uidA, rhd6 and tir1) which were inoculated with 20 µL per plate of a suspension of 1 × 1010 cell/mL; then plants were analyzed at seven days post-inoculation (dpi). Plantlets without inoculum were used as a control. In the direct treatment experiments, yeast culture was placed at the bottom of the dish five cm from the root tips of A. thaliana seedlings (10 seedlings per dish). During an indirect treatment, to avoid direct contact between plantlets and yeast culture, for which the middle segment of agar (∼2 mm thick) was entirely excised from square plates. Five plantlets were grown on the left side of the Petri dish, and the yeast culture was directly placed first from the bottom of the right side of the dish and then spread out through all the agar surfaces of the medium at the right side of the dish. Yeast-treated and untreated control dishes were incubated vertically at 22 ± 2 ºC in a growth chamber with a photoperiod of 16-h light (120 μmol m−2 s−1).
For pot inoculation assays, 4-week-old A. thaliana plants (Col-0, axr2-1, ein3, eds5, npr1-1, npr3-1, and jar1) were pre-treated by watering with 1 mL of a suspension of 6 × 1010 cells/ mL every other day for one week. Yeast cells were grown overnight in 0.67% YNB supplemented with 2% glucose and then were inoculated in 0.2X MS medium with 0.5 sucrose until the desired cell concentration. After this time, five μL droplets of B. cinerea spore suspension (5 × 104 spores/mL) were applied to the leaves surface. The parameters evaluated from B. cinerea infection, including disease incidence and measurement of lesion size, were determined at three dpi.
Evaluation of plant morphological parameters
Fresh weight, primary root length, and total chlorophyll content were determined in plantlets seven dpi. Three groups of ten plants were measured for the direct treatment, and three groups of five plantlets were used for the experiments of indirect treatment. ImageJ software was used to estimate the primary root length (cm). Plantlet fresh weight (g) was obtained using an analytical scale balance and the value obtained represents the mean of one plantlet (n = 30). Whole plants were submerged in 2 mL of 80% ethanol at room temperature in darkness for chlorophyll extraction and quantification. After 2 h, chlorophyll content was determined by measuring absorption spectra at 664 and 647 nm (Hiscox and Israelstam 1979). The concentration of chlorophyll per gram of fresh weight was calculated as follows: μMoles of chlorophyll = 7.93 (A664) + 19.53 (A647). All experiments were repeated at least three times with similar results.
Analysis of root development
To evaluate the primary root growth dynamics, we marked every 24 h the position of the root tip over the plates maintained in a vertical position. At the end of the experiment, the Petri dishes were scanned, and the root growth increments were measured using ImageJ software. From these data, the total root length was evaluated. The analysis of lateral root (LR) and LR primordium (LRP) density, length of fully elongated cells, and LR initiation index were determined on cleared roots (Dubrovsky et al. 1994). To clear the roots, we used the Malamy and Benfey protocol (1997) and some modifications described in Dubrovsky et al. (1994). The analysis of whole-mount preparations was performed using an Olympus BX53 microscope (Tokyo, Japan) equipped with differential interferential contrast (DIC; Nomarski) optics.
GUS histochemical analysis of DR5:uidA line
We placed seven-day-old DR5:uidA seedlings with yeast cells, as previously mentioned. To analyze the effect of auxin synthesis inhibitor, the 0.2X MS growth medium was supplemented with 10 μM 4-phenoxyphenylboronic acid (PPBo). After seven dpi, the DR5:uidA seedlings were subjected to GUS histochemical staining during 12 h of incubation at 37 °C in a GUS reaction buffer (0.5 mg/mL of 5-bromo-4-chloro-3-indolyl-β-D-glucuronide in 100 mM sodium phosphate, pH 7) (Jefferson et al. 1987). The tissue clarification process was performed as described by Malamy and Benfey (1997). At least 15 plantlets (five per Petri dish) were analyzed at a 10 × magnification in a Zeiss Axioskop 2 microscope for both types of treatment. All experiments were carried out at least three times with similar results. For the quantification of GUS intensity, the images were processed using the ImageJ software. The images were adjusted to the same resolution intensity, and the color intensity was measured through a threshold analysis.
Root hair analyses of the rhd6 mutant
The A. thaliana seedlings of rhd6 mutant were grown for seven days, and then plants were subjected to direct and indirect treatments performed in 0.2X MS and 0.2X MS growth medium supplemented with 50 mM CuSO4. The primary root portion from 500 to 1000 μm from the root tip was analyzed at seven dpi under 10 × magnification in a Zeiss Axioskop 2 microscope. Ten plants were analyzed for each treatment (n = 10). All experiments were performed at least three times with similar results.
Collection of Volatile Organic Compounds (VOCs) by SPME and GC − MS analysis
To identify the VOCs produced in A. thaliana-H. opuntiae co-cultivation, GC/MS analysis was conducted. A. thaliana–H. opuntiae interactions were carried out in a shared atmosphere using Petri dishes with partitioned growth medium. The experimental conditions assessed were as follows: (i) VOCs emitted by H. opuntiae growth without A. thaliana plantlets and (ii) indirect interactions when H. opuntiae was grown on MS medium. Also, as a control, the background of VOCs produced by A. thaliana and culture media MS was assessed. VOCs were extracted using a solid phase micro-extraction SPME (PDMS/DVB) 65 μM fiber (Supelco Analytical, Bellefonte, PA, USA) and analyzed according to the method described by González-Pérez et al. (2018). Before pouring the culture medium, each Petri dish was perforated with a sterile drill (2.0 mm diameter) to allow the SPME fiber entrance; three plates were analyzed for each condition. In each Petri dish, seven-day-old seedlings grown in an experiment performed with an indirect treatment design were placed on the side where the hole was made, and on the opposite side H. opuntiae was present. Next, the dishes were sealed with Parafilm® to prevent the escape of VOCs and incubated vertically for seven days at 22 ± 2 ºC in a growth chamber with a 16-h light photoperiod as described. SPME fiber was introduced into the hole and exposed for 60 min. The fiber was inserted into the injection port and desorbed for 20 min in a splitless injector at 200 °C of the gas chromatograph GC-7890b (Agilent Technologies, Santa Clara, CA, USA) coupled to a mass spectrophotometer EM-5977A (Agilent Technologies). The column used to separate the VOCs was HP-Innowax Polyethylene glycol phase capillary GC Column (30.0 m × 0.320 mm i.d. × 0.25 μm, Agilent Technologies). The column temperature conditions were set to 40 °C for 10 min, then the temperature increased at a rate of 3 °C per minute to reach a final temperature of 180 °C and held for 10 min. Helium was used as carrier gas at a constant flux rate of 1.5 mL/min. The compounds were identified by deconvolution using the W10N11 mass spectral library (Wiley10Nist11) and based on the linear retention index values (Van den Dool 1963), which were calculated after analyzing C6 and C25 n-alkanes. For each condition, the peak area of each organic compound identified was calculated as a percentage proportional to the total peak area of all volatile organic compounds. Peaks with identity lower than 90% in respect to Wiley10Nist11 library were not considered.
In vitro inhibitory assay and plant infection with B. cinerea
B. cinerea strain BMM (isolated initially from grape wine) was provided by Brigitte Mauch-Mani (University of Neuchatel, Switzerland). Both, B. cinerea in vitro growth and spore suspension preparation, were carried out as previously described by L’Haridon et al. (2011). For the in vitro inhibition assay, we used Petri dishes prepared for indirect treatment experiments. On the right side, the medium contained potato dextrose agar media (PDA) and five μL of a B. cinerea spore suspension of (5 × 104 spores/mL), and the left side contained MS medium and 20 µL of a suspension of 1 × 1010 cells/mL of H. opuntiae. The plates were incubated at 22 ± 2 ºC for 3 days.
Growth inhibition of B. cinerea was evaluated by measuring the area of the B. cinerea hyphae. The images were processed using the ImageJ software. The number of spores was determined by harvesting mycelium, which was resuspended in distilled water and filtered through glass wool to remove hyphae. Spores were 1:1000 diluted and then observed in a Neubauer chamber at a 20 × magnification in a Zeiss Axioskop 2 microscope.
B. cinerea plant infection protocol and lesion size measurement were performed as previously described by L’Haridon et al. (2011). Thus, four-week-old Arabidopsis thaliana plants were inoculated with a 1 × 1010/mL cell suspension of H. opuntiae next to the roots every other day for 1 week. After this time, five μL droplets of B. cinerea spore suspension (5 × 104 spores) were applied to the fully expanded leaves. Infection symptoms were evaluated at three dpi by measuring the disease incidence and lesion size on the leaf surface. Pictures were taken at three dpi with a digital camera. ImageJ software was used to estimate the lesion size (mm2). All experiments were performed at least three times with similar results.
Statistical analysis
All results are reported as mean values (± SE). Student’s t-test analysis (P < 0.05) was carried out to determine statistically significant differences between treatments. The software GraphPad Prism version 9.4.0 was used (2019, GraphPad Software, San Diego, CA, USA). All the data analyzed were obtained from three independent experiments.
Results
In vitro analysis of the interaction between A. thaliana and H. opuntiae
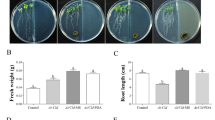
To investigate the effect of H. opuntiae on A. thaliana growth and development, we grew wild-type A. thaliana seedlings under normal conditions in the MS growth medium for seven days and then co-cultivated with H. opuntiae. After seven dpi, we recorded plant physiological parameters, such as fresh weight, chlorophyll content, and primary root growth (Fig. 1). These analyses were carried out using two different experimental conditions: a direct treatment (no physical barrier between the plants and yeast cells when the yeast culture was applied on the same agar medium surface where seedlings grew) and an indirect treatment (physical barrier: the ∼2-mm-thick agar block was removed from the middle of the plate permitting plant growth on one side of Petri dish and yeast cells on another side) (Fig. 1A, B).
A. thaliana growth and root development during direct and indirect in vitro interaction systems. Representative data of 14-day-old A. thaliana (Col-0) plantlets grown on MS media after seven dpi with H. opuntiae cells. As a control, plantlets were grown on MS medium without yeast. A, B Seedlings were exposed to yeast using A the direct interaction system (A) or the indirect interaction system without direct contact (B). In both cases, the yeast cells were distributed on the MS medium. The scale bar corresponds to 1 cm. C-E Physiological parameters were examined in shoots and roots. C Fresh weight per plant (g). D Total chlorophyll content (μMoles). E Primary root length (cm). Bar plots represent mean ± SE values of dependent variables (n = 30). Asterisks indicate a statistically significant difference between uninoculated and inoculated samples, according to the Student’s t-test (P ≤ 0.05)
Under direct treatment conditions, no plant fresh weight differences were detected when plantlets grew on a medium with H. opuntiae compared to those untreated control plantlets (Fig. 1C). In contrast, H. opuntiae indirect treatment increased the fresh weight of A. thaliana plantlets. Moreover, both types of treatment caused increased chlorophyll levels compared to the untreated control (Fig. 1D). Interestingly, we noticed a drastic alteration of root architecture in A. thaliana plants exposed to H. opuntiae, compared to untreated plantlets. Under direct treatment, the primary root length was significantly reduced (Fig. 1E) and was ~ 70% of that of the untreated control plants. In detail, we observed that the primary root growth started to delay after four days of H. opuntiae presence (Suppl. Fig. S1). In contrast, the primary root length was not different in the indirect treatment with H. opuntiae compared to the untreated control (Fig. 1E).
Since the most severe effect of yeast cells was observed during direct treatment, we selected it to explore the role of H. opuntiae in root development by analyzing the LR and LRP density and addressing whether LR initiation was affected (Fig. 2) (Malamy and Benfey 1997; Dubrovsky et al. 1994). We found that the density of LRs in a branching zone and the density of LRPs in the lateral root formation zone (see Fig. 2A bottom; Dubrovsky and Forde 2012) were greater in A. thaliana plantlets grown in a Petri dish with yeast cells in the direct experimental setup (Fig. 2A). These results suggested that in this setup, yeast promoted LR initiation (LRI) in A. thaliana primary root. This effect, however, can be a result of a decreased fully elongated cell length (Suppl. Fig. S1). To normalize the density data, we estimated the LR initiation index (ILRI), a parameter that estimates the number of LR initiation events per root portion comprising 100 cortical cells in a cell file (Dubrovsky et al. 1994). Indeed, it was found that the ILRI was 3.6 times higher in treated compared to untreated seedlings (Fig. 2B), confirming that LR initiation was indeed strongly increased by the yeast. This result suggested the auxin-like effects of the yeast cells. Commonly, auxin inhibits the primary root growth, which was also affected in this experiment (Fig. 1E). This effect, in turn, is often accompanied by a decrease in distance from the root tip to the first LR initiation event. We found that in Col-0 roots treated with H. opuntiae, this distance was also significantly decreased (Fig. 2C). Moreover, the xylem in the Col-0 treated seedling was much closer to the root tip compared to the untreated seedlings (Fig. 2D). Together these results suggest an auxin-like effect of metabolites emitted from yeast H. opuntiae cells over the root system architecture in Arabidopsis.
H. opuntiae affected the lateral root initiation in A. thaliana when plants are treated using in direct interaction system. A The lateral root (LR) density and LR primordium (LRP) density in the branching and LR formation zone. B The lateral root initiation index (LRI) estimates the number of LR initiation events in 100 cells. C Distance to the first initiated LRP in millimeters. D Representative pictures of Arabidopsis (Col-0) roots of untreated control (left) and H. opuntiae treated (right). White arrows indicate the position of the most distal differentiated xylem, which is much closer to the tip in the treated seedling (n = 18 and 20, respectively). The reference bar = 200 μm. Asterisks indicate a statistically significant difference between untreated control and H. opuntiae-treated samples, according to the Student’s t-test (P ≤ 0.05)
Auxin and ethylene responses are induced in A. thaliana during the H. opuntiae interaction
To further investigate the role of H. opuntiae in root development, we examined auxin response at a transcriptional level in A. thaliana roots using the DR5:uidA reporter line (Ulmasov et al. 1997) (Fig. 3). Seven-day-old DR5:uidA seedlings grown in Petri dishes containing H. opuntiae (at both direct and indirect treatments) showed an increase in the GUS signal in the primary root tips after seven dpi (Fig. 3A, B). Notably, in relative terms, a greater auxin response was detected after indirect treatment, which was observed with a stronger GUS signal and a greater tissue volume showing GUS activity (Fig. 3B). To confirm the effect of auxin-induced response, we applied the auxin biosynthesis inhibitor, 4-phenoxyphenylboronic acid (PPBo) (Kakei et al. 2015) to plantlets treated with the yeast (Fig. 3C, D). Accordingly, pixel intensity quantification analyses showed an increase in the GUS signal intensity in the primary root tips in the presence of H. opuntiae (Fig. 3E, F). We found that PPBo applied during plant and yeast interaction for seven days caused a decrease in the GUS signal of DR5:uidA seedlings which was reverted when treated with H. opuntiae irrespective of the type of treatment. An increased auxin response in plants treated in both ways with H. opuntiae suggested that either auxin accumulation, or local auxin synthesis, or both are enhanced in plants exposed to the yeast. Also, the auxin-sensing receptor mutant tir1 showed an increment in primary root length and fresh weight during the direct interaction conditions, while there were no differences in this parameter during indirect interaction (Fig. 3G, H), supporting the hypothesis that H. opuntiae direct interaction can lead to plant auxin-related responses.
The DR5:uidA GUS signal increases under H. opuntiae interaction. Histochemical GUS staining patterns of DR5::uidA inoculated with H. opuntiae in direct (A) and indirect (B) interaction systems. A control seedling grown on MS without any inoculation was used. C, D Pharmacological inhibition of auxin biosynthesis. Effect of 4-phenoxyphenylboronic acid (PPBo, 10 μM) during the interaction between DR5::uidA and H. opuntiae in direct (C) and indirect (D) interaction systems. Pictures represent individuals from at least 15 stained GUS seedlings for each of the interaction systems. The reference bar = 75 μm. Images of primary root tips were acquired in Zeiss Axioskop 2 microscope at 10 × magnification. E, F GUS intensity quantification (pixels/mm2) from the primary root tip in (A to D) treatments. G tir1 primary root length (cm). H tir1 fresh weight per plant (g). Bar plots represent mean ± SE values of dependent variables (n = 30). Asterisks indicate a statistically significant difference between uninoculated and inoculated samples, according to the Student’s t-test (P ≤ 0.05)
Root hairs are epidermal cell extensions that play a crucial role during nutrient acquisition, root anchorage, and environmental interactions (Vissenberg et al. 2020). It is known that, besides auxin, the phytohormone ethylene regulates root hair growth and development (Feng et al. 2017). Further, we analyzed the ethylene-associated process of root hair formation using a mutant affected in a bHLH transcription factor, ROOT HAIR DEFECTIVE 6. The rhd6 loss-of-function mutant shows a root hair impaired phenotype under normal growth conditions (Masucci et al. 1996). RHD6 protein activates another bHLH member, RHD6-LIKE 4 (RSL4), required for root hair elongation (Yi et al. 2010; Pires et al. 2013; Feng et al. 2017). When seven-day-old rhd6 mutant seedlings were inoculated with H. opuntiae yeast (both direct and indirect treatments), after seven dpi we observed that the root hair defective phenotype of the rhd6 mutant was partially reverted (Fig. 4A, B). By contrast, using the ethylene production and perception inhibitor (CuSO4) slightly delayed root hair growth in A. thaliana seedlings during both types of treatments with H. opuntiae cells (Fig. 4C, D). These data collectively suggest that during direct and indirect treatments, some diffused metabolites and some volatile compounds, respectively, emanate from H. opuntiae yeast and activate auxin- and ethylene-related responses in A. thaliana roots.
Effect of H. opuntiae in the rhd6 root hair defective mutant. Seven-day-old rhd6 mutant seedlings were inoculated with H. opuntiae cells for seven days under direct (A) and indirect (B) interaction systems. As a control, plantlets grew on MS without yeast application. C, D Pharmacological inhibition of ethylene signaling. Effect of CuSO4 (50 mM) during the interaction between H. opuntiae and rhd6 mutant under direct (C) and indirect (D) interaction systems. Images were taken under a microscope (Zeiss Axioskop 2 microscope) and represented individuals from at least 15 seedlings. Root hair images were captured approximately between 500 and 1000 μm from the primary root tip. The scale bar corresponds to 500 µm
Identification of Volatile Organic Compounds (VOCs) produced by H. opuntiae
Since we established changes in plantlet growth even without direct contact with the yeast, we aimed to identify VOCs produced by H. opuntiae cells. The VOCs were collected using a solid phase micro-extraction (SPME) for 1 h and analyzed by chromatography-mass spectrophotometry (GC/MS). We analyzed the VOC mixtures produced during A. thaliana–H. opuntiae interactions and identified six VOCs, specifically five alcohols and one aldehyde (Table 1). Notably, we identified that the most abundant VOC was 3-methyl-1-butanol, followed by ethanol in the VOCs blends (Table 1). The VOC mixtures produced by H. opuntiae when it was growing alone on the growth medium were also determined.
When we analyzed the VOC profiles emitted by H. opuntiae in the absence of the plant, we identified ten volatile compounds produced by H. opuntiae (Suppl. Table S1). The VOC mixtures encompassed mainly alcohols, among other compounds, such as acids and ketones. As observed in the split A. thaliana–H. opuntiae interaction (Table 1), the most abundant VOCs observed were 3-methyl-1-butanol and ethanol when the yeast was grown alone (Table 1). Therefore, we hypothesize that these compounds emitted by the yeast may be primarily responsible for triggering the growth promotion of A. thaliana.
H. opuntiae inhibits B. cinerea growth under in vitro conditions
Previously we determined that compounds released by H. opuntiae confer resistance to necrotrophic fungi B. cinerea (Ferreira-Saab et al. 2018). To analyze the potential role of H. opuntiae cells as a biocontrol agent, first, we determined whether H. opuntiae could inhibit the development of B. cinerea under in vitro conditions (Fig. 5A). We observed that after seven days under indirect interaction, B. cinerea inhibited its mycelial growth and decreased the number of spores in the presence of yeast cells (Fig. 5B, C). These results suggest that H. opuntiae has antifungal activity against B. cinerea.
H. opuntiae inhibits B. cinerea growth in vitro. A H. opuntiae cells (6 × 1010 cells mL−1) were placed on the upper part of a split Petri dish containing MS, while on the bottom of the Petri dish, B. cinerea spore suspension (5 × 104spores mL−1) was placed under PDA media. Plates were incubated at 22°C for 72 h. B Growth inhibition was evaluated by measuring the diameter of the B. cinerea mycelium. C Quantification of B. cinerea spores per mL. Bars represent the mean values (± SE) of three independent experiments. Asterisks indicate a statistically significant difference between uninoculated and inoculated samples, according to the Student's t-test (P ≤ 0.05)
H. opuntiae induces plant resistance to B. cinerea independently of auxin, ethylene, salicylic acid, and jasmonic acid hormonal pathways
Previously we determined that HoFs can induce systemic acquired resistance (SAR) against B. cinerea (Ferreira-Saab et al. 2018). To determine if similar protection can be achieved using H. opuntiae cells, four-week-old wild-type A. thaliana plants were inoculated with yeast cell suspension every other day for one week. Next, plants were drop inoculated with a five μl of B. cinerea spore suspension (5 × 104 spores) or water (Mock) (Fig. 6). Three dpi A. thaliana leaves inoculated with yeast cells showed disease resistance (Fig. 6A), as indicated by ~ 40% inhibition of disease incidence on H. opuntiae-treated plants compared to mock-treated ones (Fig. 6C). Moreover, the lesion size triggered by this pathogen was 50% reduced in yeast-inoculated plants than in non-inoculated plants (Fig. 6B), suggesting that H. opuntiae produced a protective effect in A. thaliana against B. cinerea pathogen infection.
H. opuntiae protects A. thaliana plants against B. cinerea infection. Four-week-old A. thaliana plants were inoculated with MS liquid medium (Mock) or H. opuntiae cells (6 × 1010 cells mL−1) for 2 weeks. A Four-week-old A. thaliana plants treated with H. opuntiae cells were infected with five μL droplets containing a B. cinerea spore suspension (5 × 104spores mL−1), and infection symptoms were evaluated at 72 hpi. Representative images of the inhibitory assay are shown. B Lesion size and C disease incidence was evaluated at 72 hpi by measuring the percentage of leaves infected per plant. Bars represent mean values (± SE) of three independent experiments, each with twenty replicates. Asterisks indicate a statistically significant difference between uninoculated and inoculated samples, according to the Student's t-test (P ≤ 0.05)
Plant hormones, including auxin, ethylene, salicylic acid, and jasmonic acid, play a central role in regulating plant defense responses. Since we determined that H. opuntiae cells can induce auxin- and ethylene-related responses (Figs. 3 and 4), we addressed whether H. opuntiae may regulate the plant resistance to B. cinerea through these phytohormone-mediated pathways. We explored if H. opuntiae also stimulated plant defense responses by salicylic acid and jasmonic acid. For this purpose, we analyzed the fungal disease incidence and lesion size in A. thaliana mutant plants impaired in auxin (axr2-1), ethylene (ein3), salicylic acid (eds5, npr1-1, and npr3-1), and jasmonic acid (jar1) pathways (Staswick et al. 1992; Timpte et al. 1994; Chao et al. 1997; Nawrath et al. 2002) during the interaction with yeast cells (Fig. 6).
As shown in Fig. 6A, four-week-old axr2-1, ein3, eds5, and jar1 plants under mock treatment showed severe disease symptoms of B. cinerea three dpi, as indicated by the high number of infected leaves and larger lesion size compared with the wild type (Fig. 6B, C). Interestingly, in axr2-1, ein3, eds5, and jar1 plants inoculated with H. opuntiae, the progression of fungal infection did not differ from WT yeast-inoculated plants (Fig. 6A). These data agree with the reduction in around 25% of disease incidence and 50% decrease of lesion size in axr2-1, ein3, eds5, and jar1 yeast-inoculated compared to non-inoculated plants (Fig. 6B, C). Lastly, npr1-1 and npr3-1 disclosed similar reductions in lesion size in the presence of H. opuntiae (Suppl. Fig. S2). These data suggest that H. opuntiae interaction can lead to plant resistance to B. cinerea independently of auxin, ethylene, salicylic acid, and jasmonic acid hormonal pathways.
Discussion
In nature, plants interact with diverse communities of microorganisms (Wang et al. 2016; Khare et al. 2018), establishing beneficial interactions, which outcome in a boost in plant growth and improving resistance and tolerance against biotic and abiotic stresses (Jones et al. 2019; Khan et al. 2021). The present study examined the interaction between A. thaliana and H. opuntiae, a fermentative yeast found on fruit surfaces (Ferreira-Saab et al. 2018; Hu et al. 2020; Saubin et al. 2020). In vitro experiments showed that H. opuntiae increased fresh weight and total chlorophyll content on A. thaliana plantlets treated in indirect mode. We established that the yeast causes substantial changes in A. thaliana root development resulting in shorter but more branched roots; these root architecture modifications resembled those triggered by auxin. In line with this, auxin reporter lines and pharmacological inhibition of auxin synthesis confirmed auxin-like effects. Moreover, these findings are supported by a previous report where H. opuntiae cells yeast exhibited the ability to produce indole-3-acetic acid (IAA) (Peng et al. 2018); however, H. opuntiae indirectly may affect plant growth by competing for both space and nutrients, since during direct interaction Arabidopsis fresh weight is reduced.
Auxin is central to plant and microorganism development and growth processes (Kunkel and Harper 2018). In plants, auxin is an important developmental regulator involved in cell division and elongation, differentiation, tropisms, apical dominance, senescence, abscission, and flowering (Woodward and Bartel 2005; Teale et al. 2006). Auxin production by a microorganism can function as a signaling molecule regulating microbial gene expression, promoting growth-form switching and potentially as a quorum-sensing molecule (Rao et al. 2010). In this respect, it is highly possible that an inhibitory effect of H. opuntiae in direct treatment experiments was caused by the auxin produced by the yeast cells, explaining the absence of a similar effect after the indirect treatment (Fig. 1E).
Pioneer reports have shown that microorganisms can regulate plant development and growth, such as in the case of Trichoderma virens, which increased biomass and promoted lateral root development in A. thaliana seedlings in an auxin-dependent manner (Contreras-Cornejo et al. 2009). In another report, González-Pérez et al. (2018) observed that the direct contact interaction between A. thaliana seedlings and Trichoderma spp abated the GUS signal in the primary root tips of the DR5:uidA reporter line. The authors suggest that fungi release secondary metabolites, decreasing the auxin accumulation, and thereby affecting the primary root growth. In contrast, we observed that the GUS signal of DR5:uidA was increased during direct interaction between A. thaliana and H. opuntiae, suggesting that microbe-produced auxin is functional, similar to one produced by plants, and produces a typical auxin response at a transcriptional level. For the moment, it is unclear how to explain an increased auxin response found after an indirect treatment with H. opuntiae (Fig. 3B, D), as diffusion of hypothetically produced auxins is not possible in this experimental setup. One possibility is that ethylene that can be produced by yeast cells (Thomas and Spencer 1978) induces auxin responses as auxin-ethylene cross-talk is well documented in A. thaliana and Trp-dependent auxin biosynthesis is upregulated by ethylene (Stepanova et al. 2005, 2007; Ivanchenko et al. 2008). However, the inhibition of root growth might indicate a hypersensitive response to diffusible compounds produced by yeast.
We observed an increase in the primary root length and fresh weight of tir1 mutant plants when they were exposed to H. opuntiae in direct interaction conditions (Fig. 3G, H), contrary to what is observed in the wild-type plants. In Arabidopsis, the F-box TIR1/AFB family of proteins that act as auxin receptors regulating the induction of the downstream nuclear signaling pathway leading to the transcriptional plant reprogramming (Watanabe et al. 2017; Die et al. 2018; Leyser 2018). It has been reported that the formation of the TIR1/AFB–Aux/IAA complex is required for root growth regulation by an independent transcriptional branch of this signaling pathway (Fendrych et al. 2018). Likely, the absence of TIR1 could lead to no effect or response during auxin application, in this sense, the primary root length and fresh weight increment in the tir1 mutant can be related to an inefficient auxin perception.
Along with ethylene, auxins are fundamental hormones controlling plant growth and development (Poupin et al. 2016). We found that H. opuntiae could restore the root hair defective phenotype of the rhd6 mutant background, which is re-established by exogenous application of auxin or ethylene (Masucci et al. 1996). Additionally, in many aspects of plant immunity and disease resistance, auxin and ethylene are regulator molecules (Llorente et al. 2008; Zhao et al. 2012; Song et al. 2014; Guan et al. 2015; Kunkel and Harper 2018).
The microorganisms produce a wide range of secondary metabolites with biological activity, including VOCs. When plants perceive microbial VOCs, they can trigger a broad-spectrum response, including plant growth promotion or inhibition, and induce plant resistance against biotic and abiotic stresses (Kanchiswamy et al. 2015; Jalali et al. 2017; Garbeva and Weisskopf 2020; Zhang et al. 2020; Ruangwong et al. 2021). The VOC emission depends on the genus and species of the microorganism as well as environmental factors such as temperature, pH, availability of nutrients, and oxygen levels (Insam and Seewald 2010; Hung et al. 2015; González-Pérez et al. 2022). Alcohols have broad effects on plants (Piechulla et al. 2017), and they represent around 16% of the VOCs produced by microorganisms (Schenkel et al. 2015). In this study, we found that during the A. thaliana-H. opuntiae interaction the VOCs identified were mainly alcohols of which the most abundant were 3-methyl-1-butanol, ethanol, and 2-methyl-1-propanol. Regarding ethanol, this compound has been reported in VOCs blends emitted by Bacillus and Trichoderma species (Farag et al. 2006; Lee et al. 2016; Wonglom et al. 2020; Elsherbiny et al. 2020). With respect to 3-methyl-1-butanol this compound also has been detected in VOCs emitted by different microorganisms which have been reported to promote plant growth (Ledger et al. 2016; Lee et al. 2016; Heenan-Daly et al. 2021; Kong et al. 2021). In this way, 3-methyl-1-butanol significantly increased fresh weight and total chlorophyll after exposure for 72 h to 3-methyl-1-butanol at 10 ng/L in A. thaliana plantlets (Lee et al. 2019). Also, seeds of maize and wheat treated with a concentration of 1 and 10 mg/L−1 of 3-methyl-1-butanol increase germination rate and seedling growth (Li et al. 2018). Evidence reported by Kong et al. (2021) and Ledger et al. (2016) shows that 3-methyl-1-butanol improves plant growth of Medicago sativa and A. thaliana under iron deficiency and salt stress.
The branched alcohol, 2-methyl-1-propanol, has been identified in VOCs blends emitted by beneficial plant-associated microorganism’s such as Bacillus, Pseudomonas, Phoma, and Trichoderma (Farag et al. 2006; Xie et al. 2009; Naznin et al. 2013; Park et al. 2015; Lee et al. 2016). The application of 2-methyl-1-propanol showed a positive effect on plant growth promotion in Arabidopsis plantlets (Lee et al. 2019). In this way, 2-methyl-propanol was found as main component in the volatile blends produced by Phoma sp. (GS8-3 strain). Tobacco plants exposed to of 2-methyl-propanol mixed with 3-methyl-1-butanol and other compounds identified showed a significant increase in the fresh weight over control condition (Naznin et al. 2013). The emission of hexyl alcohol (1-hexanol) by different rhizosphere bacteria has been reported, when Arabidopsis plantlets were exposed to this compound that showed a weak plant growth promotion (Blom et al. 2011). Additionally, was reported that fungal VOCs have been implicated in regulating A. thaliana root development and plant growth through auxin transport and signaling (González-Pérez et al. 2018; Li et al. 2022). In this study, we found that 3-methyl-1-butanol was the dominant compound in the VOC mixtures during A. thaliana–H. opuntiae interactions. Is probable that VOCs work synergistically with the other compounds here identified playing a role in growth promotion on A. thaliana when interact with H. opuntiae.
Afterward, we analyzed the resistance to B. cinerea of plants root inoculated with H. opuntiae. Previously, it was found that compounds released by the H. opuntiae (HoFs) conferred systemic protection in A. thaliana plants against B. cinerea infection (Ferreira-Saab et al. 2018). We cannot discard the idea that compounds with similar activity to HoFs can be released when A. thaliana leaves are inoculated with yeast suspension. There is also the possibility that these compounds can penetrate the leaf tissues and cells and can induce defense responses as genuine elicitors once inside the plant cell. It was already suggested that HoFs might act as elicitors inducing the pathways of jasmonic acid- and ethylene-related defense responses (Ferreira-Saab et al. 2018). Similarly, our data indicate that H. opuntiae generated plant defense responses at the systemic level, which might mediate defense signaling pathways. Remarkably, we determined that canonical responses induced by ethylene, salicylic acid (SA), and jasmonic acid (JA) apparently do not have a crucial participation in H. opuntiae-induced defense response. This opens the possibility of identifying other uncharacterized mechanisms that might be involved in the defense against B. cinerea induced by this yeast that are currently studied in our group.
Finally, we hypothesize that metabolites produced by H. opuntiae trigger plant defenses, virulence gene expression, and stress responses that limit pathogen colonization. With this in mind, H. opuntiae may represent a new eco-friendly alternative to synthetic fungicides. Since H. opuntiae is usually present during the fermentation of fruit musts, apparently, this yeast species is not associated with a harmful effect on human or animal health.
Data availability
Materials described in the manuscript, including all relevant raw data, will be freely available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality.
Abbreviations
- dpi:
-
Days post-inoculation
- HoFs:
-
H. opuntiae-Filtrates
- LR:
-
Lateral root
- LRP:
-
LR primordium
- VOC:
-
Volatile organic compound
References
Bailly A, Sovero V, Vincenzetti V et al (2008) Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J Biol Chem 283(31):21817–21826
Blom D, Fabbri C, Connor EC et al (2011) Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol 13(11):3047–3058
Bulgarelli D, Schlaeppi K, Spaepen S, Van Themaat EVL, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838
Cai J, Aharoni A (2022) Amino acids and their derivatives mediating defense priming and growth tradeoff. Curr Opin Plant Biol 69:102288
Chao Q, Rothenberg M, Solano R et al (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89(7):1133–1144
Compant S, Van Der Heijden MG, Sessitsch A (2010) Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol Ecol 73(2):197–214
Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, Lopez-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149(3):1579–1592
Contreras-Cornejo HA, Macías-Rodríguez L, Alfaro-Cuevas R, Lopez-Bucio J (2014) Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol Plant Microbe Interact 27(6):503–514
De Simone N, Pace B, Grieco F et al (2020) Botrytis cinerea and table grapes: a review of the main physical, chemical, and bio-based control treatments in post-harvest. Foods 9(9):1138
Dean R, Van Kan JA, Pretorius ZA et al (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13(4):414–430
Deveau A, Bonito G, Uehling J et al (2018) Bacterial-fungal interactions: ecology, mechanisms and challenges. FEMS Microbiol Rev 42(3):335–352
Die JV, Gil J, Millan T (2018) Genome-wide identification of the auxin response factor gene family in Cicer arietinum. BMC Genom 19(1):1–15
Donati I, Cellini A, Sangiorgio D et al (2020) Pseudomonas syringae pv. actinidiae: ecology, infection dynamics and disease epidemiology. Microb Ecol 80(1):81–102
Dubrovsky JG, Puente ME, Bashan Y (1994) Arabidopsis thaliana as a model system for the study of the effect of inoculation by Azospirillum brasilense Sp-245 on root hair growth. Soil Biol Biochem 26(12):1657–1664
Dubrovsky JG, Forde BG (2012) Quantitative analysis of lateral root development: pitfalls and how to avoid them. The Plant Cell 24(1):4–14
Effmert U, Kalderás J, Warnke R, Piechulla B (2012) Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 38(6):665–703
Elsherbiny EA, Amin BH, Aleem B et al (2020) Trichoderma volatile organic compounds as a biofumigation tool against late blight pathogen Phytophthora infestans in postharvest potato tubers. J Agric Food Chem 68(31):8163–8171
Farag MA, Ryu CM, Sumner LW, Pare PW (2006) GC–MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67(20):2262–2268
Fendrych M, Akhmanova M, Merrin J et al (2018) Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nat Plants 4(7):453–459
Feng Y, Xu P, Li B et al (2017) Ethylene promotes root hair growth through coordinated EIN3/EIL1 and RHD6/RSL1 activity in Arabidopsis. Proc Natl Acad Sci USA 114(52):13834–13839
Ferreira-Saab M, Formey D, Torres M et al (2018) Compounds released by the biocontrol yeast Hanseniaspora opuntiae protect plants against Corynespora cassiicola and Botrytis cinerea. Front Microbiol 9:1596
Fiorilli V, Catoni M, Lanfranco L, Zabet NR (2020) Editorial: Interactions of plants with bacteria and fungi: molecular and epigenetic plasticity of the host. Front Plant Sci 11:274
Garbeva P, Weisskopf L (2020) Airborne medicine: bacterial volatiles and their influence on plant health. New Phytol 226(1):32–43
González-Pérez E, Ortega-Amaro MA, Salazar-Badillo FB et al (2018) The Arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Sci Rep 8(1):16427
González-Pérez E, Ortega-Amaro MA, Bautista E et al (2022) The entomopathogenic fungus Metarhizium anisopliae enhances Arabidopsis, tomato, and maize plant growth. Plant Physiol Biochem 176:34–43
Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 39(8):1968–1977
Guan R, Su J, Meng X et al (2015) Multilayered regulation of ethylene induction plays a positive role in Arabidopsis resistance against Pseudomonas syringae. Plant Physiol 169(1):299–312
Gupta R, Elkabetz D, Leibman-Markus M et al (2021) Cytokinin drives assembly of the phyllosphere microbiome and promotes disease resistance through structural and chemical cues. ISME J 16(1):122–137
Hawkins AP, Crawford KM (2018) Interactions between plants and soil microbes may alter the relative importance of intraspecific and interspecific plant competition in a changing climate. AoB Plants 10(4):ply039
Heenan-Daly D, Coughlan S, Dillane E, Prestwich BD (2021) Volatile compounds from Bacillus, Serratia, and Pseudomonas promote growth and alter the transcriptional landscape of Solanum tuberosum in a passively ventilated growth system. Front Microbiol 12:628437
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57(12):1332–1334
Hu L, Liu R, Wang X, Zhang X (2020) The sensory quality improvement of citrus wine through co-fermentations with selected non-Saccharomyces yeast strains and Saccharomyces cerevisiae. Microorganisms 8(3):323
Ivanchenko MG, Muday GK, Dubrovsky JG (2008) Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55(2):335–347
Jalali F, Zafari D, Salari H (2017) Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecol 29:67–75
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6(13):3901–3907
Jiang CH, Xie YS, Zhu K et al (2019) Volatile organic compounds emitted by Bacillus sp. JC03 promote plant growth through the action of auxin and strigolactone. J Plant Growth Regul 87(2):317–328
Jones JD, Dangl JL (2006) The plant immune system. Nature 444(7117):323–329
Jones P, Garcia BJ, Furches A et al (2019) Plant host-associated mechanisms for microbial selection. Front Plant Sci 10:862
Kakei Y, Yamazaki C, Suzuki M et al (2015) Small-molecule auxin inhibitors that target YUCCA are powerful tools for studying auxin function. Plant J 84(4):827–837
Kanchiswamy CN, Malnoy M, Maffei ME (2015) Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front Plant Sci 6:151
Kang SM, Hamayun M, Khan MA et al (2019) Bacillus subtilis JW1 enhances plant growth and nutrient uptake of Chinese cabbage through gibberellins secretion. J Appl Bot Food Qual 92:172–178
Khan N, Ali S, Shahid MA et al (2021) Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review. Cells 10:1551
Khare E, Mishra J, Arora NK (2018) Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol 9:2732
Kidd BN, Kadoo NY, Dombrecht B et al (2011) Auxin signaling and transport promote susceptibility to the root-infecting fungal pathogen Fusarium oxysporum in Arabidopsis. Mol Plant Microbe Interact 24(6):733–748
Kong WL, Wang YH, Wu XQ (2021) Enhanced iron uptake in plants by volatile emissions of Rahnella aquatilis JZ-GX1. Front Plant Sci 12:704000
Kudjordjie EN, Sapkota R, Nicolaisen M (2021) Arabidopsis assemble distinct root-associated microbiomes through the synthesis of an array of defense metabolites. PLoS ONE 16(10):e0259171
Kunkel BN, Harper CP (2018) The roles of auxin during interactions between bacterial plant pathogens and their hosts. J Exp Bot 69(2):245–254
Lee S, Yap M, Behringer G et al (2016) Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol Biotechnol 3(1):1–14
Lee S, Behringer G, Hung R, Bennett J (2019) Effects of fungal volatile organic compounds on Arabidopsis thaliana growth and gene expression. Fungal Ecol 37:1–9
Ledger T, Rojas S, Timmermann T, Pinedo I, Poupin MJ, Garrido T, Richter P, Tamayo J, Donoso R (2016) Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front Microbiol 7:1838
Leyser O (2018) Auxin signaling. Plant Physiol 176(1):465–479
L’Haridon F, Besson-Bard A, Binda M et al (2011) A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity. PLoS Pathog 7(7):e1002148
Li C, Zhang J, Zhao C et al (2018) Effects of 3-methyl-1-butanol on seed germination and seedling growth of maize and wheat. Bot Res 38(5):785–789
Li P, Lu YJ, Chen H, Day B (2020) The lifecycle of the plant immune system. Crit Rev Plant Sci 39(1):72–100
Li Y, Shao J, Fu Y et al (2022) The volatile cedrene from Trichoderma guizhouense modulates Arabidopsis root development through auxin transport and signalling. Plant Cell Environ 45(3):969–984
Llorente F, Muskett P, Sánchez-Vallet A et al (2008) Repression of the auxin response pathway increases Arabidopsis susceptibility to necrotrophic fungi. Mol Plant 1(3):496–509
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124(1):33–44
Masucci JD, Schiefelbein JW (1996) Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. The Plant Cell 8(9):1505–1517
Mansfield J, Genin S, Magori S et al (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13(6):614–629
Mikiciński A, Sobiczewski P, Puławska J, Malusa E (2016) Antagonistic potential of Pseudomonas graminis 49M against Erwinia amylovora, the causal agent of fire blight. Arch Microbiol 198:531–539
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Nawrath C, Heck S, Parinthawong N, Métraux JP (2002) EDS5, an essential component of salicylic acid–dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14(1):275–286
Naznin HA, Kimura M, Miyazawa M, Hyakumachi M (2013) Analysis of volatile organic compounds emitted by plant growth-promoting fungus Phoma sp. GS8–3 for growth promotion effects on tobacco. Microbes Environ 28(1):42–49
Nieto-Jacobo MF, Steyaert JM, Salazar-Badillo FB et al (2017) Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front Plant Sci 8:102
Park YS, Dutta S, Ann M et al (2015) Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem Biophys Res Comm 461(2):361–365
Peng X, Wang Y, Tang LJ et al (2018) Yeasts from Nanfeng mandarin plants: occurrence, diversity and capability to produce indole-3-acetic acid. Biotechnol Equip 32(6):1496–1506
Piechulla B, Lemfack MC, Kai M (2017) Effects of discrete bioactive microbial volatiles on plants and fungi. Plant Cell Environ 40(10):2042–2067
Pires ND, Yi K, Breuninger H et al (2013) Recruitment and remodeling of an ancient gene regulatory network during land plant evolution. Proc NAS 110(23):9571–9576
Poupin MJ, Greve M, Carmona V, Pinedo I (2016) A complex molecular interplay of auxin and ethylene signaling pathways is involved in Arabidopsis growth promotion by Burkholderia phytofirmans PsJN. Front Plant Sci 7:492
Rao RP, Hunter A, Kashpur O, Normanly J (2010) Aberrant synthesis of indole-3-acetic acid in Saccharomyces cerevisiae triggers morphogenic transition, a virulence trait of pathogenic fungi. Genetics 185(1):211–220
Ruangwong OU, Wonglom P, Suwannarach N, Kumla J, Thaochan N, Chomnunti P, Pitija K, Sunpapao A (2021) Volatile organic compound from Trichoderma asperelloides TSU1: Impact on plant pathogenic fungi. J Fungus 7(3):187
Saubin M, Devillers H, Proust L et al (2020) Investigation of genetic relationships between Hanseniaspora species found in grape musts revealed interspecific hybrids with dynamic genome structures. Front Microbiol 10:2960
Schenkel D, Lemfack MC, Piechulla B, Splivallo R (2015) A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Front Plant Sci 6:707
Song S, Huang H, Gao H et al (2014) Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26(1):263–279
Staswick PE, Su W, Howell SH (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89(15):6837–6840
Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17(8):2230–2242
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19(7):2169–2185
Teale WD, Paponov IA, Palme K (2006) Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7(11):847–859
Thomas KC, Spencer M (1978) Evolution of ethylene by Saccharomyces cerevisiae as influenced by the carbon source for growth and the presence of air. Can J Microbiol 24(6):637–642
Timpte C, Wilson AK, Estelle M (1994) The axr2-1 mutation of Arabidopsis thaliana is a gain-of-function mutation that disrupts an early step in auxin response. Genetics 138(4):1239–1249
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell 9(11):1963–1971
Vishwakarma K, Kumar N, Shandilya C et al (2020) Revisiting plant–microbe interactions and microbial consortia application for enhancing sustainable agriculture: a review. Front Microbiol 11:3195
Vissenberg K, Claeijs N, Balcerowicz D, Schoenaers S (2020) Hormonal regulation of root hair growth and responses to the environment in Arabidopsis. J Exp Bot 71(8):2412–2427
Wang K, Sipilä TP, Overmyer K (2016) The isolation and characterization of resident yeasts from the phylloplane of Arabidopsis thaliana. Sci Rep 6(1):1–13
Watanabe E, Mano S, Hara-Nishimura I et al (2017) HSP90 stabilizes auxin receptor TIR1 and ensures plasticity of auxin responses. Plant Signal Behav 12(5):e1311439
Wonglom P, Ito SI, Sunpapao A (2020) Volatile organic compounds emitted from endophytic fungus Trichoderma asperellum T1 mediate antifungal activity, defense response and promote plant growth in lettuce (Lactuca sativa). Fungal Ecol 43:100867
Xie X, Zhang H, Paré PW (2009) Sustained growth promotion in Arabidopsis with long-term exposure to the beneficial soil bacterium Bacillus subtilis (GB03). Plant Signal Behav 4:948–953
Yi K, Menand B, Bell E, Dolan L (2010) A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet 42(3):264–267
Zhang H, Kaushal R, Singh SK, Paré P (2020) Bacterial volatile-mediated plant abiotic stress tolerance. In: Ryu CM, Weisskopf L, Piechulla B (eds) Bacterial volatile compounds as mediators of airborne interactions. Springer, Singapore, pp 187–200
Zhao Y, Wei T, Yin KQ et al (2012) Arabidopsis RAP2.2 plays an important role in plant resistance to Botrytis cinerea and ethylene responses. New Phytol 195(2):450–460
Zimmerli L, Stein M, Lipka V, Somerville S (2004) Host and non-host pathogens elicit different jasmonate/ethylene responses in Arabidopsis. Plant J 40(5):633–646
Acknowledgements
The authors thank Martha Torres for her technical assistance. I.M-L acknowledges a postdoctoral fellowship from Dirección General de Asuntos del Personal Académico-UNAM No. CJIC/CTIC/1077/2018. N.Y.A-B., Y.J.R.-C., and E.J.F.-C. have received a Mexican Consejo Nacional de Ciencia y Tecnología (CONACyT) fellowships No. 929445, 745733 and 1137328, respectively. This work was supported by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT)-UNAM grants IN203720 (to M.S.) and IN204221 (to J.G.D.) and CONACyT grant A1-S-9236 (to J.G.D.).
Author information
Authors and Affiliations
Contributions
IM-L, YJR-C, KRFS-E, JGD, JFJB, and MS conceived and designed the experiments. IM-L, YJR-C, SN-M, EG-P, NYA-B, AICM, and EJF-C performed the experiments. IM-L, YJR-C, EG-P, JGD, JFJB, and MS analyzed the data and wrote and revised the paper All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this study was conducted without any commercial relationships that could lead to any potential conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
425_2023_4326_MOESM1_ESM.tiff
Supplementary Fig. S1 Root growth kinetics. The Arabidopsis primary root length (cm) measurements were recorded in A. thaliana seedlings treated with H. opuntiae and untreated control in both direct (A) and indirect (B) experimental conditions. Bar plots represent mean ± SE values of dependent variables (n = 10 and 5, respectively). C Length of fully elongated cells in µm in untreated and H. opuntiae-treated samples (n = 18 and 20, respectively). Bars represent the mean values (± SE) of three independent experiments. Asterisks indicate a statistically significant difference between uninoculated and inoculated samples, according to the Student's t-test (P ≤ 0.05)
425_2023_4326_MOESM2_ESM.tiff
Supplementary Fig. S2 H. opuntiae protects A. thaliana plants against B. cinerea infection. Four-week-old (Col-0, npr1-1, and npr3-1) A. thaliana plants were inoculated with MS liquid medium (Mock) or H. opuntiae cells (6x1010 cells mL−1) for two weeks. A Four-week-old A. thaliana plants treated with H. opuntiae cells were infected with five μL droplets containing a B. cinerea spore suspension (5x104spores mL−1), and infection symptoms were evaluated at 72 hpi. Representative images of the inhibitory assay are shown. B Lesion size was evaluated at 72 hpi by measuring the percentage of leaves infected per plant. Bars represent mean values (± SE) of three independent experiments, each with twenty replicates. Asterisks indicate a statistically significant difference between uninoculated and inoculated samples, according to the Student's t-test (P ≤ 0.05)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maruri-López, I., Romero-Contreras, Y.J., Napsucialy-Mendivil, S. et al. A biostimulant yeast, Hanseniaspora opuntiae, modifies Arabidopsis thaliana root architecture and improves the plant defense response against Botrytis cinerea. Planta 259, 53 (2024). https://doi.org/10.1007/s00425-023-04326-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04326-6