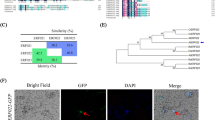
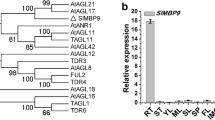
Abstract
Main conclusion
SlGCC, a GARP transcription factor, functions as a root-related transcriptional repressor. SlGCC synchronizes auxin and ethylene signaling involving SlPIN3 and SlIAA3 as intermediate targets sketching a molecular map for lateral root development in tomato.
Abstract
The root system is crucial for growth and development of plants as it performs basic functions such as providing mechanical support, nutrients and water uptake, pathogen resistance and responds to various stresses. SlGCC, a GARP family transcription factor (TF), exhibited predominant expression in age-dependent (initial to mature stages) tomato root. SlGCC is a transcriptional repressor and is regulated at a transcriptional and translational level by auxin and ethylene. Auxin and ethylene mediated SlGCC protein stability is governed via proteasome degradation pathway during lateral root (LR) growth development. SlGCC over-expressor (OE) and under-expressed (UE) tomato transgenic lines demonstrate its role in LR development. This study is an attempt to unravel the vital role of SlGCC in regulating tomato LR architecture.









Similar content being viewed by others
Data availability
All data generated and/ or analyzed in this study are included in the manuscript and its supplementary information.
Change history
09 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00425-024-04371-9
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- CDS:
-
Coding sequence
- DAG:
-
Days after germination
- GCC:
-
GARP and coiled-coil
- LR:
-
Lateral root
- OE:
-
Over-expressor
- PR:
-
Primary root
- RSA:
-
Root System Architecture
- TF:
-
Transcription factor
- UE:
-
Under-expressed
References
Abas L, Kolb M, Stadlmann J et al (2021) Naphthylphthalamic acid associates with and inhibits PIN auxin transporters. Proc Natl Acad Sci USA 118(1):e2020857118
Alarcón MV, Lloret PG, Salguero J (2014) Synergistic action of auxin and ethylene on root elongation inhibition is caused by a reduction of epidermal cell length. Plant Signal Behav 9(3):e28361
Argyros RD, Mathews DE, Chiang YH et al (2008) Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell 20(8):2102–2116
Bassa C, Mila I, Bouzayen M, Audran-Delalande C (2012) Phenotypes associated with down-regulation of Sl-IAA27 support functional diversity among Aux/IAA family members in tomato. Plant Cell Physiol 53(9):1583–1595
Bhalerao RP, Eklöf J, Ljung K et al (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29(3):325–332
Chaabouni S, Jones B, Delalande C et al (2009) Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth. J Exp Bot 60(4):1349–1362
De Smet I (2010) Multimodular auxin response controls lateral root development in Arabidopsis. Plant Signal Behav 5(5):580–582
Deng W, Yang Y, Ren Z et al (2012) The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol 194(2):379–390
Du Y, Scheres B (2018) Lateral root formation and the multiple roles of auxin. J Exp Bot 69(2):155–167
Eshed Y, Baum SF, Perea JV, Bowman JL (2001) Establishment of polarity in lateral organs of plants. Curr Biol 11(16):1251–1260
Gattolin S, Alandete-Saez M, Elliott K et al (2006) Spatial and temporal expression of the response regulators ARR22 and ARR24 in Arabidopsis thaliana. J Exp Bot 57(15):4225–4233
Hall LN, Rossini L, Cribb L, Langdale JA (1998) GOLDEN 2: a novel transcriptional regulator of cellular differentiation in the maize leaf. Plant Cell 10(6):925–936
Hawker NP, Bowman JL (2004) Roles for class III HD-Zip and KANADI genes in Arabidopsis root development. Plant Physiol 135(4):2261–2270
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular Circ – Calif Agric Exp Stn 347(2nd edn)
Hu Y, Vandenbussche F, Van Der Straeten D (2017) Regulation of seedling growth by ethylene and the ethylene–auxin crosstalk. Planta 245:467–489
Ivanchenko MG, Muday GK, Dubrovsky JG (2008) Ethylene–auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55(2):335–347
Iwabuchi K, Li B, Bartel P, Fields S (1993) Use of the two-hybrid system to identify the domain of p53 involved in oligomerization. Oncogene 8(6):1693–1696
Janiak A, Kwaśniewski M, Szarejko I (2016) Gene expression regulation in roots under drought. J Exp Bot 67(4):1003–1014
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6(13):3901–3907
Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS (2001) KANADI regulates organ polarity in Arabidopsis. Nature 411(6838):706–709
Khan K, Kumar V, Niranjan A, Shanware A, Sane VA (2019) JcMYB1, a Jatropha R2R3MYB transcription factor gene, modulates lipid biosynthesis in transgenic plants. Plant Cell Physiol 60(2):462–475
Kiba T, Inaba J, Kudo T, Ueda N et al (2018) Repression of nitrogen starvation responses by members of the Arabidopsis GARP-type transcription factor NIGT1/HRS1 subfamily. Plant Cell 30(4):925–945
Kumar V, Singh D, Majee A, Singh S, Asif MH, Sane AP, Sane VA (2021) Identification of tomato root growth regulatory genes and transcription factors through comparative transcriptomic profiling of different tissues. Physiol Mol Biol Plant 27(6):1173–1189
Lavenus J, Goh T, Roberts I, Guyomarc’h S et al (2013) Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18(8):450–458
Lewis DR, Negi S, Sukumar P, Muday GK (2011) Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 138(16):3485–3495
Li Y, Liu X, Chen R, Tian J, Fan Y, Zhou X (2019) Genome-scale mining of root-preferential genes from maize and characterization of their promoter activity. BMC Plant Biol 19:584
Liberman LM, Sparks EE, Moreno-Risueno MA, Petricka JJ, Benfey PN (2015) MYB36 regulates the transition from proliferation to differentiation in the Arabidopsis root. Proc Natl Acad Sci USA 112(39):12099–12104
Lin D, Yang Y, Khalil R, Xian Z, Hu G, Li Z (2013) SlmiR393 controls the auxin receptor homologous genes expression, and regulates sensitivity to auxin in tomato root growth. Sci Hortic 162:90–99
Liu M, Chen Y, Chen Y, Shin JH, Mila I et al (2018) The tomato Ethylene Response Factor Sl-ERF. B3 integrates ethylene and auxin signaling via direct regulation of Sl-Aux/IAA 27. New Phytol 219(2):631–640
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Ma J, Wang LY, Dai JX, Wang Y, Lin D (2021) The NAC-type transcription factor CaNAC46 regulates the salt and drought tolerance of transgenic Arabidopsis thaliana. BMC Plant Biol 21(1):1–11
Majee A, Kumar V, Bano N, Kumari A, Bag SK, Sane VA (2022) Elucidation of heat shock transcription factor family (HSFs) postulates significant insights for the identification of their putative roles in root development and hormonal regulation in tomato. J Plant Growth Regul 42:2327–2344
MakinoS KT, Imamura A, Hanaki N, Nakamura A et al (2000) Genes encoding pseudo-response regulators: insight into His-to-Asp phosphorelay and circadian rhythm in Arabidopsis thaliana. Plant Cell Physiol 41(6):791–803
McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R, Fraley R (1986) Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant Cell Rep 5:81–84
Medici A, Marshall-Colon A, Ronzier E, Szponarski W et al (2015) AtNIGT1/HRS1 integrates nitrate and phosphate signals at the Arabidopsis root tip. Nat Commun 6(1):6274
Mehra P, Pandey BK, Melebari D, Banda J, Leftley N, Couvreur V, Rowe J, Anfang M, De Gernier H, Morris E, Sturrock CJ (2022) Hydraulic flux–responsive hormone redistribution determines root branching. Science 378(6621):762–768
Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17(4):181–195
Negi S, Ivanchenko MG, Muday GK (2008) Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 55(2):175–187
Negi S, Sukumar P, Liu X, Cohen JD, Muday GK (2010) Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J 61(1):3–15
Nibau C, Gibbs DJ, Coates JC (2008) Branching out in new directions: the control of root architecture by lateral root formation. New Phytol 179(3):595–614
Nilsson L, Müller R, Nielsen TH (2010) Dissecting the plant transcriptome and the regulatory responses to phosphate deprivation. Physiol Plant 139(2):129–143
Park J, Lee Y, Martinoia E, Geisler M (2017) Plant hormone transporters: what we know and what we would like to know. BMC Plant Biol 15:93
Péret B, De Rybel B, Casimiro I, Benková E, Swarup R et al (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14(7):399–408
Péret B, Middleton AM, French AP, Larrieu A et al (2013) Sequential induction of auxin efflux and influx carriers regulates lateral root emergence. Mol Syst Biol 9(1):699
Qin H, Huang R (2018) Auxin controlled by ethylene steers root development. Int J Mol Sci 19(11):3656
Qin H, Zhang Z, Wang J, Che X, Wei P, Huang R (2017) The activation of OsEIL1 on YUC8 transcription and auxin biosynthesis is required for ethylene-inhibited root elongation in rice early seedling development. PLOS Genet 13(8):e1006955
Ren Z, Wang X (2016) SlTIR1 is involved in crosstalk of phytohormones, regulates auxin-induced root growth and stimulates stenospermocarpic fruit formation in tomato. Plant Sci 253:13–20
Riechmann JL, Heard J, Martin G, Reuber L et al (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290(5499):2105–2110
Ron M, Kajala K, Pauluzzi G, Wang D et al (2014) Hairy root transformation using Agrobacterium rhizogenes as a tool for exploring cell type-specific gene expression and function using tomato as a model. Plant Physiol 166(2):455–469
Rosado D, Ackermann A, Spassibojko O, Rossi M, Pedmale UV (2022) WRKY transcription factors and ethylene signaling modify root growth during the shade-avoidance response. Plant Physiol 188(2):1294–1311
Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15(16):2122–2133
Růžička K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19(7):2197–2212
Safi A, Medici A, Szponarski W, Ruffel S, Lacombe B, Krouk G (2017) The world according to GARP transcription factors. Curr Opin Plant Biol 39:159–167
Shin R, Burch AY, Huppert KA, Tiwari SB, Murphy AS, Guilfoyle TJ, Schachtman DP (2007) The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 19(8):2440–2453
Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17(8):2230–2242
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19(7):2169–2185
Street IH, Aman S, Zubo Y, Ramzan A et al (2015) Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiol 169(1):338–350
Swarup R, Perry P, Hagenbeek D, Van Der Straeten D et al (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19(7):2186–2196
Swarup K, Benková E, Swarup R, Casimiro I et al (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10(8):946–954
Veloccia A, Fattorini L, Della Rovere F, Sofo A et al (2016) Ethylene and auxin interaction in the control of adventitious rooting in Arabidopsis thaliana. J Exp Bot 67(22):6445–6458
Wang S, Tiwari SB, Hagen G, Guilfoyle TJ (2005) AUXIN RESPONSE FACTOR7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts. Plant Cell 17(7):1979–1993
Wang S, Chang Y, Guo J, Chen JG (2007) Arabidopsis Ovate Family Protein 1 is a transcriptional repressor that suppresses cell elongation. Plant J 50(5):858–872
Wang Z, Zheng Z, Song L, Liu D (2018) Functional characterization of Arabidopsis PHL4 in plant response to phosphate starvation. Front Plant Sci 9:1432
Wang ZY, Zhao S, Liu JF, Zhao HY et al (2022) Genome-wide identification of Tomato Golden 2-Like transcription factors and abiotic stress related members screening. BMC Plant Biol 22(1):82
Wieczorek P, Wrzesińska B, Obrępalska-Stęplowska A (2013) Assessment of reference gene stability influenced by extremely divergent disease symptoms in Solanum lycopersicum L. J Virol Methods 194(1–2):161–168
Woeste KE, Ye C, Kieber JJ (1999) Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol 119(2):521–530
Wolters H, Jürgens G (2009) Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet 10(5):305–317
Wykoff DD, Grossman AR, Weeks DP, Usuda H, Shimogawara K (1999) Psr1, a nuclear localized protein that regulates phosphorus metabolism in Chlamydomonas. Proc Natl Acad Sci USA 96(26):15336–15341
Yokoyama A, Yamashino T, Amano YI, Tajima Y, Imamura A, Sakakibara H, Mizuno T (2007) Type-B ARR transcription factors, ARR10 and ARR12, are implicated in cytokinin-mediated regulation of protoxylem differentiation in roots of Arabidopsis thaliana. Plant Cell Physiol 48(1):84–96
Zhang L, Qin LN, Zeng ZR, Wu CZ, Gong YY, Liu LH, Cao FQ (2019) Molecular identification of a root apical cell-specific and stress-responsive enhancer from an Arabidopsis enhancer trap line. Plant Methods 15(1):1–11
Zourelidou M, Absmanner B, Weller B, Barbosa IC et al (2014) Auxin efflux by PIN-FORMED proteins is activated by two different protein kinases, D6 PROTEIN KINASE and PINOID. Elife 3:e02860
Acknowledgements
We would like to extend gratitude to Prof. Shucai Wang (Northeast Normal University, Changchun, China) for vectors. The authors are thankful to Dr. Mondher Bouzayen (Toulouse, France) for sharing the tomato (Ailsa craig cultivar) seeds. Mr. Ram Awadh for handling and taking care of the transgenics in glasshouse. Funding by CSIR, Delhi (BSC204 and MLP007) is acknowledged. This manuscript represents the CSIR-NBRI communication number CSIR-NBRI_MS/2023/09/01.
Funding
VK and SBT received financial support for this study from CSIR (GOI), AM from DST-INSPIRE(GOI), PP from CSIR funded project MLP007, VAS from CSIR funded BSC204 and MLP007 project.
Author information
Authors and Affiliations
Contributions
VK experiments, data analysis, manuscript writing; AM data analysis and manuscript writing; PP and BS experiments and discussion; APS conceptualized and discussion; VAS experiment designing, data analysis, discussion, manuscript writing and fund acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors affirm no competing interests.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, V., Majee, A., Patwal, P. et al. A GARP transcription factor SlGCC positively regulates lateral root development in tomato via auxin-ethylene interplay. Planta 259, 55 (2024). https://doi.org/10.1007/s00425-023-04325-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04325-7