Abstract
Main conclusion
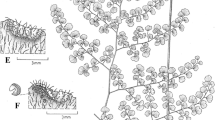
The cytological changes underlying the formation of an apoplasmic barrier in the multi-layered extrafloral nectaries of Citharexylum myrianthum are compatible with the synthesis, transport and deposition of suberin. In terms of ontogenesis and function, the intermediate layers of these nectaries are homologous with the stalks of nectar-secreting trichomes.
Abstract
Anticlinal cell wall impregnations are common in trichomatic nectaries and their functions as endodermis-like barriers have been discussed because of possible direct effects on the nectary physiology, mainly in the nectar secretion and resorption. However, the cytological events linked to nectary wall impregnations remain little explored. This study documents the ontogenesis and the fine structure of the EFN cells, and cytological events linked to the wall impregnations of multi-layered extrafloral nectaries (EFNs) in Citharexylum myrianthum Cham. (Verbenaceae). EFNs are patelliform, and differentiated into (a) a multicellular foot, which is compound in structure and vascularised with phloem strands, (b) a bi-layered intermediate region with thickened cell walls and (c) a single-layered secretory region with palisade-like cells. EFNs are protodermal in origin, starting with a single protodermal cell and ending with the complex, multi-layered structure. The cell wall impregnations first appear in the very young EFN and increase towards maturity. Lipid patches (assumed to be suberin) are deposited on the inner faces of the primary walls, first along the anticlinal walls and then extend to the periclinal walls. On both walls, plasmodesmata remain apparently intact during the maturation of the EFNs. In the peripheral cytoplasm there are abundant polymorphic plastids, well-developed Golgi bodies often close to rough endoplasmic reticulum profiles, mitochondria and polyribosomes. Cytological events linked to the wall impregnations are consistent with suberin synthesis, transport and deposition. Our findings offer new insights into the structure-properties of specialised nectary cell walls and so should contribute to our knowledge of the physiological and protective roles of this structure in nectar glands.







Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are provided in full in the results section of this paper.
References
Beattie AJ (1985) The evolutionary ecology of ant-plant mutualisms. Cambridge University Press, Cambridge
Beisson F, Li YH, Bonaventure G, Pollard M, Ohlrogge JB (2007) The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19:351–368
Beisson F, Li-Beisson Y, Pollard M (2012) Solving the puzzles of cutin and suberin polymer biosynthesis. Curr Opin Plant Biol 15:329–337
Bernards MA (2002) Demystifying suberin. Can J Bot 80:227–240
Bernards MA, Razem FA (2001) The poly(phenolic) domain of potato suberin: a non-lignin cell wall bio-polymer. Phytochemistry 57:1115–1122
Bernards MA, Lopez ML, Zajicek J, Lewis NG (1995) Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin. J Biol Chem 270:7382–7386
Bigs AR (1984) Intracellular suberin: occurrence and detection in tree bark. IAWA Bull 5:243–248
Bigs AR (1985) Detection of impervious tissue in tree bark with selective histochemistry and fluorescence microscopy. Stain Technol 60:299–304
Cardoso-Gustavson P, Davis AR (2015) Is nectar reabsorption restricted by the stalk cells of floral and extrafloral nectary trichomes? Plant Biol 17:134–146
Chavan AR, Deshmukh YS (1964) Studies in organogeny and ontogeny -IV. A study of the floral organogeny and ontogeny of extrafloral nectaries in the genus Clerodendrum J.M.S. Univ Baroda 13:27–29
Christensen NM, Faulkner C, Oparka K (2009) Evidence for unidirectional flow through plasmodesmata. Plant Physiol 150:96–104
Clair-Maczulajtys D, Bory G (1983) Les enctaries extrafloraux pédicellés chez Ailanthus glandulosa. Can J Bot 61:683–691
Cohen H, Szymanski JJ, Aharoni A (2017) Assimilation of ‘omics’ strategies to study the cuticle layer and suberin lamellae in plants. J Exp Bot 68:5389–5400
Cohen H, Fedyuk V, Wang C, Wu S, Aharoni A (2020) SUBERMAN regulates developmental suberization of the Arabidopsis root endodermis. Plant J 102:431–447
Davis AR, Peterson RL, Shuel RW (1988) Vasculature and ultrastructure of the floral and stipular nectaries of Vicia faba (Leguminosae). Can J Bot 66:1435–1448
Donaldson S (2020) Autofluorescence in plants. Molecules 25:2393. https://doi.org/10.3390/molecules25102393
Dop P (1928) Les glandes de Clerodendrum foetidum Bunge. Bull Soc His Nat Toulouse 57:167–169
Durkee LT, Baird CW, Cohen PF (1984) Light and electron microscopy of the resin glands of Passiflora foetida (Passifloraceae). Am J Bot 71:596–602
Eleftheriou EP, Hall JL (1983) The extrafloral nectaries of cotton. I. Fine structure of the secretory papillae. J Exp Bot 34:103–119
El-Gazzar A, Watson L (1970) A taxonomic study of labiatae and related genera. New Phytol 69:45–486
Elias T (1983) Extrafloral nectaries: their structure and distribution. In: Bentley B, Elias T (eds) The biology of nectaries. Columbia University Press, New York, pp 174–203
Enstone DE, Peterson CA (1997) Suberin deposition and band plasmolysis in the corn (Zea mays L.) root exodermis. Can J Bot 75:1188–1199
Enstone DE, Peterson CA, Ma F (2003) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21:335–351
Epel BL (1994) Plasmodesmata: composition, structure and trafficking. Plant Mol Biol 26:1343–1356
Erbar C (2014) Nectar secretion and nectaries in basal angiosperms, magnoliids and non-core eudicots and a comparison with core eudicots. Plant Div Evol 131:63–143
Fahn A (1979a) Secretory tissues in plants. Academic Press, London
Fahn A (1979b) Ultrastructure of nectaries in relation to nectar secretion. Am J Bot 66:977–985
Fahn A (2000) Structure and function of secretory cells. In: Hallahan DL, Gray JC (eds) Advances in botanical research. Plant trichomes. Academic Press, New York, pp 37–75
Franke R, Schreiber L (2007) Suberin—a biopolyester forming apoplastic barriers. Curr Opin Plant Biol 10:252–259
Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L (2005) Apoplastic polyesters in Arabidopsis surface tissues: a typical suberin and a particular cutin. Phytochemistry 66:2643–2658
Gou JY, Yu XH, Liu CJ (2009) A hydroxycinnamoyl transferase responsible for synthesizing suberin aromatics in Arabidopsis. Proc Natl Acad Sci USA 106:18855–18860
Graça J (2015) Suberin: the biopolyester at the frontier of plants. Front Chem 3:62. https://doi.org/10.3389/fchem.2015.00062
Graski S, de Feijter AW, Schindler M (1983) Endoplasmic reticulum forms a dynamic continuum for lipid diffusion between contiguous soybean root cells. Plant Cell 5:25–38
Gunning BES, Hughes JE (1976) Quantitative assessment of symplastic transport of pre-nectar into the trichomes of Abutilon nectaries. Aust J Plant Physiol 3:619–637
Inamdar JA (1969) Structure and ontogeny of foliar nectaries and stomata in Bignonia chamberlaynii Sims. Proc Indian Acad Sci Section B 70:232–240
Jensen WA (1962) Botanical histochemistry. Freeman, San Francisco, W.H
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Kirk PW Jr (1970) Neutral red as a lipid fluorochrome. Stain Technol 45:1–4
Koptur S (1992) Extrafloral nectary-mediated interactions between insects and plants. In: Bernays E (ed) Insect–plant interactions. CRC Press, Boca Raton, pp 81–129
Kraus JE, Sousa HC, Rezende MH, Castro NM, Vecchi C, Luque R (1998) Astra blue and basic fuchsin double staining of plant materials. Biotech Histochem 73:235–243
Schreiber L, Breine H-W, Riederer M, Duggelin M, Guggenheim R (1994) The Casparian strip of Clivia miniata reg. roots: isolation, fine structure and chemical nature. Bot Acta 107:353–361
Li Y, Beisson F, Koo AJ, Molina I, Pollard M, Ohlrogge J (2007) Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc Natl Acad Sci USA 104:18339–18344
Liu LS, Chen GY, Fishman ML, Hicks KB (2005) Pectin gel vehicles for controlled fragrance delivery. Drug Deliv 12:149–157
Lüttge U (1971) Structure and function of plant glands. Ann Rev Plant Physiol 22:23–44
Ma F, Peterson CA (2001) Development of cell wall modifications in the endodermis and exodermis of Allium cepa roots. Can J Bot 79:621–634
Ma F, Peterson CA (2003) Current insights into the development, structure and chemistry of the endodermis and exodermis. Can J Bot 81:405–421
Machado SR, Rodrigues TM (2019) Autophagy and vacuolar biogenesis during the nectary development. Planta 250:519–533
Machado SR, Morellato LPC, Sajo MG, Oliveira OS (2008) Morphological patterns of extrafloral nectaries in woody plant species of the Brazilian cerrado. Plant Biol 10:660–673
Machado SR, Gregório EA, Rodrigues TM (2018) Structural associations between organelle membranes in nectary parenchyma cells. Planta 247:1067–1076
Maheshwari JK (1954) The structure and development of extra-floral nectaries in Duranta plumieri Jacq. Phytomorphology 4:208–211
Maheshwari JK, Chakrabarty B (1966) Foliar nectaries of Clerodendrum japonicum (Thumb.) sweet. Phytomorphology 16:75–80
Marazzi B, Bronstein JL, Koptur S (2013) The diversity, ecology and evolution of extrafloral nectaries: current perspectives and future challenges. Ann Bot 111:1243–1250
McDade LA, Turner MD (1997) Structure and development of bracteal nectary glands in Aphelandra (Acanthaceae). Am J Bot 84:1–15
McFarlane HE, Watanabe Y, Yang W, Huang Y, Ohlrogge J, Samuels AL (2014) Golgi- and trans-Golgi network-mediated vesicle trafficking is required for wax secretion from epidermal cells. Plant Physiol 164:1250–1260
Metcalfe CR, Chalk L (1950) Anatomy of dicotyledons, vol 1. Clarendon, Oxford
Metcalfe CR, Chalk L (1979) Anatomy of the dicotyledons: systematic anatomy of leaf and stem with a brief history of the subject, vol 1, 2nd edn. Clarendon, Oxford
Meyer CJ, Peterson CA (2011) Casparian bands occur in the periderm of Pelargonium hortorum stem and root. Ann Bot 107:591–598
Molina I, Li-Beisson Y, Beisson F, Ohlrogge JB, Pollard M (2009) Identification of an Arabidopsis feruloyl-coenzyme A transferase required for suberin synthesis. Plant Physiol 151:1317–1328
Nawrath C, Schreiber L, Franke RB, Geldner N, Pinto JJR, Kunst L (2013) Apoplastic diffusion barriers in Arabidopsis. Arabidopsis Book. https://doi.org/10.1199/tab.0167
Nepi M (2007) Nectary structure and ultrastructure. In: Nicolson SW, Pacini E, Nepi M (eds) Nectaries and nectar. Springer, Dordrecht, pp 129–166
O’Brien TP, Feder N, McCully ME (1964) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373
Olmstead RG, Bremer B, Scott KM, Palmer JD (1993) A parsimony analysis of the Asteridae sensu lato based on rbcL sequences. Ann Mo Bot Gard 80:700–722
Padma Rao PP, Ramayya N (1992) Structure and distribution of extrafloral nectaries (EFN) in Clerodendrum L. (Verbenaceae). J Indian I Sci 72:131–137
Paiva EAS (2009) Ultrastructure and post-floral secretion of the pericarpial nectaries of Erythrina speciosa (Fabaceae). Ann Bot 104:937–944
Pate JS, Peoples MB, Storer PJ, Atkins CA (1985) The extrafloral nectaries of cowpea (Vigna unguiculata (L.). Walp): II. Nectar composition, origin of nectar solutes and nectary functioning. Planta 166:28–38
Perumalla CJ, Peterson C (1986) Deposition of Casparian bands and suberin lamellae in the exodermis and endodermis of young corn and onion roots. Can J Bot 64:1873–1878
Reinecke M, Walther C (1978) Aspects of turnover and biogenesis of synaptic vesicles at locust neuromuscular junctions as revealed by iodide-osmium tetroxide (ZIO) reacting with intravesicular SH-groups. J Cell Biol 21:839–855
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–212
Robards AW, Stark M (1988) Nectar secretion in Abutilon: a new model. Protoplasma 142:79–91
Rocha JF, Machado SR (2009) Anatomy, ultrastructure and secretion of Hibiscus pernambucensis Arruda (Malvaceae) extrafloral nectary. Rev Bras Bot 32:489–498
Roeser KR (1972) Die nadel der schwarzkiefer-massenprodukt und kunstwerk der natur. Mikrokosmos 61:33–36
Sawidis T, Eleftheriou EP, Tsekos I (1987) The floral nectaries of Hibiscus rosa-sinensis. I. Development of the secretory hairs. Ann Bot 59:643–652
Schreiber L (2010) Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci 15:546–553
Scott MG, Peterson RL (1979) The endodermis in Ranunculus acris. I. Structure and ontogeny. Can J Bot 57:1040–1062
Subramanian RB, Inamdar JA (1985) Occurrence, structure, ontogeny, and biology of nectaries in Kigelia pinnata DC. Bot Mag Tokyo 98:67–73
Subramanian RB, Inamdar JA (1986) Nectaries in Bignonia illicium: ontogeny, structure and function. Proc Indian Acad Sci Plant Sciences 96:135–140
Subramanian RB, Inamdar JA (1989) The structure, secretion and biology of nectaries in Tecomaria capensis Thunb. (Bignoniaceae). Phytomorphology 39:69–74
Vandoorn WG, Stead AD (1997) Abscission of flowers and floral parts. J Exp Bot 48:821–837
Vassilyev AE (2010) On the mechanisms of nectar secretion: revisited. Ann Bot 10:349–354
Vishwanath SJ, Delude C, Domergue F, Rowland O (2014) Suberin: biosynthesis, regulation, and polymer assembly of a protective extracellular barrier. Plant Cell Rep 34:573–586
Weber M, Keeler KH (2013) The phylogenetic distribution of extrafloral nectaries in plants. Ann Bot 111:1251–1261
Acknowledgements
We thank the National Council for Scientific and Technological Development—CNPq for financial support (Process number 401053/2016-4 and for the research Grants to T. M. Rodrigues (Process number 303981/2018-0) and S. R. Machado (Process number 308982/2020-7); and the staff of Centro de Microscopia Eletrônica (CME), IBB, UNESP, for helping with sample preparation. We are grateful to Eliete Correa Soares for help with in loco photography, and to Dr. Sandy Lang (Rescript.co.nz) for helping with the review and editing of the English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any conflict of interest.
Additional information
Communicated by Anastasios Melis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Machado, S.R., Rodrigues, T.M. Apoplasmic barrier in the extrafloral nectary of Citharexylum myrianthum (Verbenaceae). Planta 254, 19 (2021). https://doi.org/10.1007/s00425-021-03663-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03663-8