Abstract
Main conclusion
The transfer of polyunsaturated fatty acids from phosphatidylcholine to other lipids involves several enzymes. In Camelina sativa seeds, acyl-CoA:lysophosphatidylcholine acyltransferases could be one of the most important players in this process.
Abstract
The transfer of polyunsaturated fatty acids from the location of their synthesis (phosphatidylcholine) to other lipids, e.g., triacylglycerol, remains insufficiently understood. Several enzymes could be involved in this process. One of these enzymes is acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs). In Camelina sativa seeds, LPCATs could be one of the most important players in this process. Our data clearly indicate that the CsLPCATs present in developing seeds have the potential to transfer almost all polyunsaturated fatty acids synthesised on phosphatidylcholine to the acyl-CoA pool. CsLPCAT activity is the highest at 30 °C, and the enzymes operate well at a pH of 7.0–11.0, with the best activity at a pH of 9.0. The activity of CsLPCATs was inhibited by calcium and magnesium ions at a concentration of 0.05–2 mM. In the forward reaction, CsLPCATs preferentially utilise 18:2-CoA; however, other C18 unsaturated fatty acids are also well accepted. In the backward reactions, there is no clear discrimination between the C18 unsaturated fatty acids utilised by the enzymes for phosphatidylcholine remodelling. The activity of CsLPCATs does not differ much between the stages of seed development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Camelina sativa L. Crantz (false flax or gold of pleasure), a member of the Brassicaceae family, is a relict oil crop that has been cultivated since the Bronze Age. Until World War II, C. sativa was one of the most important oilseed crops. However, after the war, false flax was displaced in Europe by other higher-yielding crops such as rapeseed and sunflower (Zubr 1997). In recent years, interest in C. sativa has grown again as its oil represents a potentially new source of essential fatty acids for the human diet, particularly omega-3 fatty acids. Over 50% of the fatty acids in C. sativa oil are polyunsaturated fatty acids with a health beneficial ratio between 18:2 and 18:3 (approximately 1:2). This oil also contains a low amount of saturated fatty acids (approximately 10%) and a high content of antioxidant, especially vitamin E (over 70 mg/100 g). All of these factors make C. sativa oil an attractive component of the human diet (Zubr 1997; Putnam et al. 1993; Rodriguez-Rodriguez et al. 2013). Additionally, C. sativa is a promising oilseed crop for the genetic manipulation of oil content and oil quality, e.g., for industrial use. The low acreage of C. sativa cultivation makes it easy to separate eventual transgenic cultivars from non-transformed cultivars.
Acyl-CoA:lysophospholipid acyltransferases (LPLATs) are commonly occurring enzymes in plants, animals and microorganisms. In the forward reaction, LPLATs use lysophospholipids and acyl-CoAs to synthesise the corresponding phospholipids. These enzymes can also transfer fatty acids from phospholipids to the acyl-CoA pool via reverse reactions. Different LPLATs could possess different substrate specificities towards fatty acid acceptors and donors both in their forward and reverse reactions. Depending on the specificity towards the fatty acid acceptor (lysophospholipids), these enzymes take a different names (Shindou et al. 2009; Lager et al. 2013; Jasieniecka-Gazarkiewicz et al. 2016). The enzymes with the highest specificity for lysophosphatidylcholine are called acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs). To date, plant LPCAT activity has been found in microsomal fractions isolated from different plants, e.g., from the developing seeds of safflower (Carthamus tinctorius L.) (Ichihara et al. 1995), sunflower (Helianthus annuus) (Fraser and Stobart 2000), soybean (Glycine max L.) (Tumaney and Rajasekharan 1999) and rapeseeds (Brasica napus) (Zheng et al. 2012). The genes encoding LPCAT were first cloned from Arabidopsis thaliana in 2008 (Ståhl et al. 2008), and cDNA encoding these enzymes has also been identified in B. napus (Zheng et al. 2012), Nicotiana benthamiana (Zhang et al. 2015), Linum usitatissimum (Pan et al. 2015), Hiptage benghalensis, Lesquerella fendleri, Ricinus communis and C. tinctorius (Lager et al. 2013).
In oilseeds, the fatty acids synthesised from acetyl-CoA in the plastids are exported to the cytosol for triacylglycerol (TAG) assembly in the endoplasmic reticulum (ER) (Browse and Somerville 1991). The newly synthesised fatty acids, mainly oleic acid (18:1) and a small amount of palmitic acid (16:0) and stearic acid (18:0), could be either directly used for diacylglycerol (DAG) esterification to produce TAG via the action of diacylglycerol acyltransferase (DGAT) or could be first incorporated into phosphatidylcholine (PC) for further modifications (Kennedy 1961). The oleic acid in PC is further desaturated to form polyunsaturated linoleic (18:2) and α-linolenic (18:3) acids by the fatty acid desaturases FAD2 and FAD3 associated with the ER. The synthesised polyunsaturated fatty acids in PC could be transferred to the cytosolic pool of acyl-CoA available for TAG synthesis, e.g., via the backward reaction of LPCATs (Lager et al. 2013; Jasieniecka-Gazarkiewicz et al. 2016). The modified fatty acids in PC could also enter the TAG synthesis pathway via the action of CDP-choline:diacylglycerol cholinephosphotransferase (CPT), phosphatidylcholine:diacylglycerol cholinephosphotransferase (PDCT) or phospholipid:diacylglycerol acyltransferase (PDAT). The first two enzymes provide diacylglycerol with modified fatty acids in PC for TAG biosynthesis, and the third enzyme can directly transfer these modified fatty acids from PC to diacylglycerol, producing TAG (Slack et al. 1983; Banaś et al. 2000; Dahlqvist et al. 2000; Bates et al. 2012). The 18:1-CoA exported from plastids could also be elongated to 20:1 and 22:1 by fatty acid elongases before incorporation into TAG (Li-Beisson et al. 2013).
However, thus far, the relative contribution of the enzymes presented above to the transfer of polyunsaturated fatty acids from PC to TAG has not been sufficiently characterised as the activities and substrate specificities of these enzymes have been studied in detail only in a narrow group of selected oilseed crops.
For example, the activity and substrate specificity of the LPCAT type of enzymes have not been studied in C. sativa. To bridge this knowledge gap, we characterised the biochemical properties of LPCATs in microsomal fractions from developing C. sativa seeds. Moreover, after optimising the assay conditions, we determined the activity and substrate specificity of these enzymes. We have also made predictions regarding the possible contribution of LPCATs to the transfer of polyunsaturated fatty acids from PC to TAG. These predictions were made by comparing the in vivo TAG accumulation intensity and acyl exchange intensity between the PC and acyl-CoA pools obtained from in vitro assays (which allowed us to characterise the backward reaction capacity of LPCATs present in the microsomal fraction from developing C. sativa seeds at different stages of development).
Materials and methods
Chemicals
[1-14C]-labelled fatty acids were purchased from Perkin Elmer (Waltham, MA, USA), and non-radioactive fatty acids and sn-1-18:1-lysophosphatidylcholine were purchased from Larodan (Malmö, Sweden). Free CoA, bovine serum albumin (BSA) and heptadecanoic acid methyl ester (17:0-Me) were supplied by Sigma-Aldrich (St. Louis, MO, USA). The standards for thin-layer chromatography were obtained from Avanti Polar Lipids (Alabaster, AL, USA). The acyl-CoAs and [1-14C]-labelled acyl-CoAs were prepared according to the modified method described by Sánchez et al. (1973). The other biochemicals and solvents used for analysis were from Merck (Darmstadt, Germany) or Sigma-Aldrich.
Plant materials
The analyses were performed on C. sativa L. Crantz, cv. Suneson. The plants were grown in a growth chamber at a constant temperature of 23 °C with 60% relative humidity and the following long day photoperiod: 16 h of light/8 h of dark at a light intensity of 120 µmol photons m−2 s−1. Plants with well-developed flower buds were marked, and after 10, 17, 24, 31 and 60 days, parts of their siliques were harvested. The seeds were manually removed from the siliques, and their fresh and dry weights were measured. The freshly harvested seeds were also used to isolate microsomal fractions and for lipid analysis.
Lipid analyses
Lipid extraction from the seeds of C. sativa was performed according to the modified methods described by Bligh and Dyer (1959). The seeds were homogenised in Potter–Elvehjem homogeniser with 3.75 ml of chloroform:methanol (1:2; v:v) with subsequent addition of 1.25 ml of 0.15 M acetic acid, 1.25 ml of chloroform and 1.25 ml of water. After vigorous mixing and centrifugation, the bottom chloroform fractions (containing lipids) were collected, dried under a stream of N2 and dissolved in 1 ml of chloroform.
To analyse the individual lipid classes and determine their fatty acid content and composition, aliquots of the obtained chloroform fractions were separated by thin-layer chromatography on silica gel 60 plates (Merck), using hexane:diethyl ether:acetic acid (70:30:1; v:v:v) or, in the case of PC content analyses, chloroform:methanol:acetic acid:water (90:15:10:2,5; v:v:v:v) as the solvent system. The separated lipid classes were visualised by a brief exposure to I2 vapours and identified by means of standards. Marked gel fragments containing the appropriate lipid classes were removed and transmethylated in situ on a gel by adding 2 ml of 2% H2SO4 in dry methanol (40 min at 90 °C). After incubation, an internal standard (heptadecanoic acid methyl ester) was added together with 3 ml of hexane and 2 ml of water. Following vigorous shaking and centrifugation, the hexane fractions, containing fatty acid methyl esters, were collected and analysed by gas chromatography (Shimadzu; GC-2010) equipped with a flame ionization detector (FID) and a 60 m × 0.25 mm CP-WAX 58-CB fused-silica column (Agilent Technologies; Santa Clara, CA, USA).
To analyse the fatty acid content and composition of the total acyl lipids present in the chloroform extracts, aliquots of these extracts were dried under a stream of N2, transmethylated and analysed with GC as described above.
Preparation of microsomal membrane
For the isolation of the microsomal fractions, three stages of seed development were chosen, 17, 24 and 31 DAF, in which it was possible to manually separate the embryo from the seed coat. The collected seed embryos were placed in glass homogeniser and ground after adding 0.1 M potassium phosphate buffer (pH 7.2) containing 1 mg/ml bovine serum albumin, 0.33 M sucrose and catalase (1000 U/ml). The homogenates were filtered through two layers of Miracloth, diluted with fresh extraction buffer to 20 ml and centrifuged at 20,000×g for 12 min. The obtained supernatants were collected and centrifuged again at 100,000×g for 90 min. The resulting pellets (microsomal fractions) were washed with 0.1 M potassium phosphate buffer (pH 7.2) and homogenised with a small volume of potassium buffer. All stages of microsomal membrane preparation were conducted at 4 °C, and the final isolated fractions were stored at − 80 °C until further analysis. To determine the membrane concentrations in the obtained microsomal fractions, aliquots of the suspensions were used for phosphatidylcholine (PC) content analyses.
Enzyme assays
Optimisation tests were carried out to establish the best conditions for the in vitro assays that determined the activity and substrate specificity of the LPCATs from C. sativa seeds in forward reactions. Five factors: reaction time, temperature, buffer pH, amount of microsomal fraction and selected ion (K+, Ca2+, Mg2+) concentration, were analysed. In all of these assays, 5 nmol of exogenous sn-1-18:1-lysophosphatidylcholine and 5 nmol of [14C]18:1-CoA were used as exogenous substrates. The results obtained from the preceding tests were included in successive analyses.
After the optimisation step, the activity and substrate specificity of the LPCATs at three different stages of C. sativa seed development (17, 24 and 31 DAF) were studied. Ten different [14C]-labelled acyl-CoAs were used: decanoyl-CoA ([14C]10:0-CoA), lauroyl-CoA ([14C]12:0-CoA), myristoyl-CoA ([14C]14:0-CoA), palmitoyl-CoA ([14C]16:0-CoA), stearoyl-CoA ([14C]18:0-CoA), oleoyl-CoA ([14C]18:1-CoA), linoleoyl-CoA ([14C]18:2-CoA), linolenoyl-CoA ([14C]18:3-CoA), eicosenoyl-CoA ([14C]20:1-CoA) and erucoyl-CoA ([14C]22:1-CoA). The reaction mixtures contained 5 nmol of exogenous sn-1-18:1-lysophosphatidylcholine, 5 nmol of appropriate [14C]acyl-CoA and aliquots of microsomal fractions equivalent to 0.2 nmol of endogenous PC (approximately 0.88 µg of microsomal proteins) in 100 µl of 40 mM potassium buffer (pH 7.2) or 40 mM HEPES buffer (pH 7.2; assays testing the effects of the selected ion concentrations on LPCAT activity). The reactions were carried out at 30 °C for 30 min with shaking (1250 rpm). The reactions were terminated by the addition of 375 μl of chloroform:methanol (1:2; v:v), 5 µl of glacial acetic acid, 125 μl of chloroform and 125 μl of water. After vigorous shaking, the samples were centrifuged, and chloroform fractions were collected. The extracted lipids present in the chloroform fractions were separated by thin-layer chromatography on silica gel 60 plates (Merck) using chloroform:methanol:acetic acid:water (90:15:10:2,5; v:v:v:v) as the solvent system. The reaction products ([14C]-PC) were visualised and quantified using electronic autoradiography (Instant Imager, Packard Instrument Co.).
In the case of substrate selectivity assays, only the microsomal fractions of C. sativa seeds from the “third” stage of development (31 DAF) were used. In these types of assays, five different acyl donors (1 nmol of each) were added to the reaction mixtures (one of the acyl donors was [14C]-labelled). The experiments were performed under two conditions: (i) without the addition of BSA (as in all experiments concerning the forward reaction of CsLPCATs) and (ii) with the addition of BSA, 0.2 mg/assay. All other analysis steps were performed as described above for the assays with only one acyl-CoA in the incubation buffer.
To determine the positional specificity of the LPCATs in the developing seeds of C. sativa in forward reactions, the ether analogue of sn-1-18:1-LPC, 1-O-9-cis-octadecenyl-sn-glycero-3-phosphocholine (sn-1-O-GPC), and the ether analogue of sn-2-18:1-LPC, 2-O-9-cis-octadecenyl-sn-glycero-3-phosphocholine (sn-2-O-GPC) were used as acyl acceptors together with [14C]18:1-CoA, [14C]18:2-CoA or [14C]16:0-CoA as the acyl donor. The LPC ether analogues in these assays were chosen as the naturally existing sn-2-LPC (fatty acid is esterified to glycerol backbone) is unstable, and acyl groups rapidly migrate to the sn-1 position, contrary to the ether analogue of sn-2-LPC (Lager et al. 2013). The microsomal fractions of seeds at 24 DAF were used for the assays. All other analysis steps were performed as described above.
The activity and substrate specificity of LPCATs in the developing seeds of C. sativa in backward reactions were examined according to the method described by Jasieniecka-Gazarkiewicz et al. (2016). Incubations were carried out in the presence of aliquots of microsomal fractions corresponding to 5 nmol of endogenous PC (approximately 22 µg of microsomal proteins), 10 nmol of [14C]acyl-CoA, 0.2 µmol of free coenzyme A (CoA) and 1 mg of BSA in a total volume of 100 µl of 40 mM potassium buffer (pH 7.2) with or without 0.5 µmol of dithionitrobenzoic acid (DTNB). Reactions were terminated after 60 min of incubation, and the reaction products were analysed as described above. The activity of the backward reactions catalysed by the LPCATs present in aliquots of the tested microsomal fractions was calculated by subtracting the amount of products synthesised in the assays with DTNB (CoA is bound by DTNB and the backward reaction is stopped) from the amount of products (de novo synthesised [14C]PC) of the assays performed without DTNB (Jasieniecka-Gazarkiewicz et al. 2016; Schemes 1 and 2).
The reactions catalysed by acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs). In the forward reaction, the synthesis of phosphatidylcholine (PC) is catalysed by the transfer of acyl groups from acyl-CoA to lysophosphatidylcholine (LPC); in the backward reaction, the transfer of acyl groups from PC to coenzyme A (CoA) and synthesis of acyl-CoA takes place
Measurement of the backward reaction activity of CsLPCATs in seed microsomal fractions. In each case, two types of assays were performed: one without and one with the addition of DTNB (compound that binds to CoA and makes it unavailable for LPCAT, therefore stopping the backward reaction). Bottom chart presents data from the reactions with microsomal fractions of C. sativa seeds at 17 DAF and exogenous [14C]18:1-CoA
Results
Lipid accumulation in C. sativa seeds
Analyses began at 10 DAF and continued at 7-day intervals until 31 DAF. Additional analyses were performed at 60 DAF when the seeds reached complete maturity. The average dry weight of individual seeds at the outset of the analyses was approximately 0.14 mg and increased by approximately 10 times at maturity, achieving 1.4 mg/seed. Between 10 and 31 DAF, the increase in seed dry weight was almost linear, with the exception of the period between 17 and 24 DAF, when it was slightly slower. Between 31 DAF and maturity, the seed dry weight increased by approximately 0.3 mg–—approximately 21% of the final seed dry weight (Fig. 1).
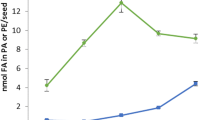
The first samples of developing C. sativa seeds were collected for lipid analyses at 10 DAF. At that point in time, the lipid accumulation in seeds was small—below 5% of the final amount of lipids present in mature seeds. Between 10 and 17 DAF, the lipid content in the seeds increased to approximately 16% of the final amount. The highest intensity of lipid accumulation occurred between 17 and 24 DAF, when over 50% of lipids were accumulated. Another intensive lipid accumulation process took place between 24 and 31 DAF when over 30% of the total lipids were synthesised. After 31 DAF, there was no net increase in lipid content (Fig. 2).
Accumulation of total acyl lipids and triacylglycerols (main component of acyl lipids) in developing C. sativa seeds. The amount of fatty acids transferred from PC to the acyl-CoA pool via the backward reaction catalysed by CsLPCATs (calculated based on backward reaction activity in vitro) is also presented. Mean values and SD are given (n ≥ 3)
TAG was the dominant lipid class in C. sativa seeds, except for the first stage of development. At 10 DAF, the relative amount of TAG was slightly less than the amount of polar lipids and accounted for approximately 42% of the total lipids. At the later stages, the relative amount of TAG gradually increased, reaching approximately 93% of all lipids at maturity. The amount of polar lipids was relatively high at the early stages of seed development (approximately 45% of total lipids) but accounted for only 3.7% of the total lipids in the mature seeds. The relative amount of DAG ranged from 2.1% (10 DAF) to 0.6% (mature seeds). The relative amount of free fatty acids decreased from approximately 7.5% (10 and 17 DAF) to 1.4% (mature seeds) of the total lipids. The amount of sterol esters fluctuated between 1 and 1.8%, with no clear trend among the analysed stages of seed development. MAG contents were the lowest among all lipid classes and ranged between 0.2 and 0.3% of the total lipids (except for stage 1–10 DAF, where MAG accounted for approximately 1% of the total lipids) (Table 1).
Despite the decrease in the relative amount of polar lipids in C. sativa developing seeds over time, their absolute amount increased until 24 DAF. Afterwards, the absolute amount decreased, especially between 24 and 31 DAF. Phosphatidylcholine—the major phospholipid—accounted for approximately 53% of the total polar lipids. The changes in the absolute amount of PC in the developing seeds reflect the changes in total polar lipids (Fig. 3).
The analysis of the fatty acid composition of the total acyl lipids present in chloroform extracts of C. sativa seeds revealed thirteen different types of them. Only five of these fatty acids (16:0; 18:0; 18:1; 18:2 and 18:3) were observed in detectable amounts at 10 DAF, and all the remaining fatty acids began to appear in the later stages of seed development. Six of them (20:0, 20:2, 20:3, 22:0, 24:0, 24:1) were present only in small amounts, and they were analysed together as “other” fatty acids (Table 2). Linoleic (18:2) and linolenic (18:3) acids were the dominant fatty acids throughout the entire seed development period. However, the relative amount of 18:2 decreased from approximately 44% at 10 DAF to approximately 18% at seed maturity, while conversely, 18:3 levels increased from approximately 12% to approximately 37%. Monounsaturated oleic acid (18:1∆9) and eicosenoic acid (20:1∆11) were the second most prevalent group of fatty acids in terms of the amount found in C. sativa seeds. The highest amount of 18:1 was at 17 DAF (23%), and 18:1 accounted for approximately 12% of all the fatty acids in mature seeds. The relative amount of 20:1 accounted for approximately 5% at 17 DAF and 13–15% in other stages of seed development. The relative amount of saturated fatty acids decreased throughout seed development: 16:0 decreased from approximately 23% (10 DAF) to 7.5% at maturity and 18:0 decreased from approximately 6% to approximately 3%. Erucic acid (22:1∆13) was detected at 17 DAF (0.5%), and its level increased during seed development to approximately 3% in mature seeds. The amount of “other” fatty acids also increased during seed development from 2.4% (17 DAF) to 6.6% in mature seeds (Table 2). The fatty acid composition of the analysed lipid classes reflected, to a certain degree, the composition of fatty acids described above (data not presented). Nevertheless, in the “polar lipid” class, erucic acid and “other” fatty acids were not present, and the relative amount of 20:1 ranged only between 2 and 4%. The relative amount of 16:0 was high (ranging from 17 to 24%), and the level of 18:3 had substantially decreased between 31 DAF (45%) and maturity (26%) (Table 3).
In vitro activity of C. sativa LPCATs
In the experiments, [14C]18:1-CoA and sn-1-18:1-LPC were used as exogenous substrates for LPCATs and the microsomal fractions of C. sativa seeds at 24 DAF were used as a source of enzymes. In the preliminary assays under conditions adapted from earlier studies (Lager et al. 2013; Jasieniecka-Gazarkiewicz et al. 2016), we showed that the reaction rate catalysed by the LPCATs in the analysed microsomal fractions was almost linear up to 30 min of incubation. These preliminary results were confirmed in further assays under optimised assays conditions (Fig. 4a).
The effect of various factors on the activity of acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) of C. sativa seeds. a Time dependency. b Temperature dependency. c pH dependency. d Microsomal content dependency. Mean values and SD are presented (data from at least three independent assays)
Aliquots of microsomal fractions containing 0.2 nmol of microsomal PC (approximately 0.88 µg of microsomal proteins) were optimal for the assays. Either higher or lower amounts of these fractions resulted in lower enzyme activity (Fig. 4d). The optimal temperature for the LPCATs of C. sativa seeds was shown to be 30 °C. Nevertheless, enzyme activity was rather high at temperatures ranging between 20 and 40 °C. Decreasing the temperature to 10 °C reduced the enzyme activity to approximately 1/3 of the maximum level. Increasing the temperature to 50 °C almost inactivated the enzymes; their activity plunged to approximately 1/7 of the maximal level. At 60 °C, the enzyme activity was negligible (Fig. 4b).
Four buffers were used to determine the effect of pH: 0.1 M phosphate buffer (pH 5.5–8.0); 0.1 M Tris–HCl buffer (pH 8.0–10.0); 0.1 M NaHCO3–NaOH buffer (pH 10.0 and 11.0); and 0.1 M NaHPO4–NaOH buffer (pH 11.0 and 12.0). C. sativa LPCATs were inactive at a pH of 5.5, and up to a pH of 6.0, their activity increased only marginally (1/10 of the maximum activity). The enzymes operated fairly efficiently at a pH of 7.0–11.0. The maximum activity of LPCATs was observed at a pH of 9.0 (Tris–HCl buffer). Above that level, LPCAT activity decreased to approximately 64% at a pH of 11.0 (NaHCO3–NaOH buffer) and to approximately 34% of its maximal value at a pH of 12.0 (Na2HPO4–NaOH buffer) (Fig. 4c).
The LPCAT activity of C. sativa seeds was also affected by the concentration of Mg+2, Ca+2 and K+ ions. In these assays, HEPES buffer was used instead of phosphate buffer (in which Mg+2 and Ca+2 ions form insoluble salts). The addition of magnesium and calcium ions to the incubation buffer at concentrations as low as 0.05 mM inhibited LPCAT activity by 27–34%. An increase in the tested ion concentrations further strengthened the LPCAT inhibition. However, the inhibition rate was not linearly correlated with increase in ion concentrations, at least in the case of Mg+2 and Ca+2. At a concentration of 2 mM of the tested ions in the assay buffer, the inhibition rate ranged from approximately 28% in the case of K+ to 53% (Mg+2) and approximately 62% (Ca+2) (Fig. 5).
The effect of various ions on the activity of acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) of C. sativa seeds. a Effect of magnesium ions. b Effect of calcium ions. c Effect of potassium ions. Mean values and SD are presented (data from at least three independent assays). Asterisks denote significant differences between the control (ions not added) and the tested ion concentrations in a mean difference two-sided Student’s t test: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001
The LPCATs in C. sativa seed microsomal fractions were rather stable enzymes. Preincubation of microsomal fractions at 30 °C for 60 min reduced LPCAT activity by approximately 38% and preincubation for approximately 15 min reduced the activity by only approximately 16% (Fig. 6).
The effect of preincubation time on the activity of the acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) in C. sativa seeds. Mean values and SD are presented (data from at least three independent assays). Asterisks denote significant differences between the activity of the CsLPCATs in the non-preincubated microsomal fraction (control) and those preincubated for different times at 30 °C in a mean difference two-sided Student’s t test: *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001
Heating the tested microsomal fractions for 10 min at 100 °C completely destroyed all LPCAT activity (data not presented).
Activity and substrate specificity of the LPCATs in developing C. sativa seeds in forward reactions
LPCAT activity was measured in microsomal fractions prepared from C. sativa seeds at 17 DAF, 24 DAF and 31 DAF, i.e., at the time points of seed development when lipids were the most intensively accumulated. In the assays, ten different [14C]acyl-CoAs in combination with sn-1-18:1-LPC were used as exogenous substrates. For the majority of the tested substrate combinations, the highest LPCAT activity was observed in the microsomal fractions prepared from the seeds at 24 DAF (except for 10:0-CoA and 18:0-CoA, which showed the lowest activity at this time point, and 20:1-CoA and 22:1-CoA, which were not accepted by CsLPCATs). Nonetheless, the LPCAT activity in the two other stages of seed development was also high and usually amounted to over 50% of the levels observed at 24 DAF (Fig. 7). The most preferred acyl donor in the assays with the microsomal fraction of the 24 and 31 DAF seeds was 18:2-CoA (at 17 DAF 18:3-CoA was preferred). The maximum LPCAT activity with this acyl-CoA reached approximately 210 pmol [14C]PC/nmol of microsomal PC/min (equal to 52 nmol [14C]PC/mg microsomal protein/min). The two other unsaturated 18C-acyl-CoAs (18:1 and 18:3) were also well accepted by the enzymes. The efficiency of CsLPCATs towards 18:1-CoA and 18:3-CoA was between 60 and 130% of its activity towards 18:2-CoA depending on the developmental stage of the seed. 16:0-CoA and 18:0-CoA were utilised with efficiencies ranging from approximately 12–28% and 4–12% of activity towards 18:2-CoA, respectively. The enzyme also utilised 10:0-CoA, 12:0-CoA and 14:0-CoA, however, with a very low activity (below 6% of its activity towards 18:2-CoA) (Fig. 7).
The substrate selectivity assays were performed to verify whether the preferences of CsLPCATs towards different acyl-CoAs observed in the assays described above were similar when different acyl-CoAs were in the reaction mixture. In these assays, five different acyl-CoAs (1 nmol of each) were added to the reaction mixtures (one acyl-CoA was [14C]-labelled), and all other steps of the analysis were carried out in the same way as in the case of the assays with only one acyl-CoA. The results showed that the preferences of CsLPCATs towards different acyl-CoAs added as an equimolar mixture were very similar to those obtained in the assays with only one acyl-CoA. 18:2-CoA was the most favoured substrate, whereas 18:3-CoA and 18:1-CoA were utilised with approximately 30% lower efficiency. The incorporation of acyl groups from 16:0-CoA and 18:0-CoA was relatively weak (approximately 6 and 1% of activity towards 18:2-CoA, respectively), similar to the single acyl-CoA assay (Fig. 8a). The addition of BSA to the incubation buffer at a concentration of 0.2 mg/assay slightly increased the utilisation of 16:0-CoA, 18:0-CoA and 18:1-CoA compared to 18:2-CoA (to 16%, 6% and 80%, respectively) and decreased the utilisation of 18:3-CoA to approximately 45% of the utilisation of 18:2-CoA (Fig. 8b).
Activity of the acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) in C. sativa seeds towards five different acyl-CoAs added to the reaction mixture together in equimolar concentrations without (a) or with (b) the addition of BSA (forward reaction; substrate selectivity assay). For the assays, the microsomal fractions of 31 DAF seeds were used. Mean values and SD are presented (data from at least three independent assays)
The assays with the ether analogue of sn-1-18:1-LPC (sn-1-O-GPC) and the ether analogue of sn-2-18:1-LPC (sn-2-O-GPC) showed that the CsLPCATs in seed microsomal fractions can acylate both LPC positions. However, the efficiency of CsLPCATs towards the sn-2 position was approximately 8 times higher than that towards the sn-1 position when 18:1-CoA and 18:2-CoA were used as fatty acid donors and approximately1.6 times higher when 16:0-CoA was used (Fig. 9).
Activity of C. sativa LPCATs towards the ether analogue of sn-1-18:1-LPC (sn-1-18:1-O-GPC) and the ether analogue of sn-2-18:1-LPC (sn-2-18:1-O-GPC) with [14C]16:0-CoA, [14C]18:1-CoA and [14C]18:2-CoA as acyl donors. For the assays, the microsomal fractions of the seeds from the stage with the highest activity of LPCATs (24 DAF) was used. Mean values and SD are presented (data from at least three independent assays)
Activity of the LPCATs in developing C. sativa seeds in backward reactions
Similar to the measurement of CsLPCAT activity in the forward reactions, we also measured the activity of these enzymes in the backward reactions using microsomal fractions prepared from C. sativa seeds at 17 DAF, 24 DAF and 31 DAF. In these assays, three [14C]acyl-CoAs (18:1-CoA, 18:2-CoA and 18:3-CoA) were used as fatty acid donors for remodelling endogenous/microsomal PC. Reactions were carried out with and without the addition of DTNB. We assumed that in both types of reactions, the incorporation of [14C]acyl groups into PC would occur via the acylation of endogenous LPC. However, the endogenous LPC in assays with DTNB was created only via reactions other than the LPCAT backward reactions (DTNB binds CoA and the backward reaction was inhibited), e.g., PDAT or lipase activity, while in assays without DTNB, LPC was synthesised in the mentioned above “other reactions” and additionally via the backward reaction of LPCATs. Thus, by subtracting the amount of de novo synthesised [14C]PC in assays with DTNB from the amount of [14C]PC synthesised in the assays without DTNB, we obtained the activity of the backward reaction of LPCATs (Jasieniecka-Gazarkiewicz et al. 2016, Scheme 2).
In our assays, the activity of the backward reactions of the CsLPCATs in seed microsomal fractions was generally the highest at 17 DAF. There was a clear difference in the reaction intensities among the analysed stages of seed development when 18:1-CoA was used as a fatty acid donor for PC remodelling. At 24 and 31 DAF, the activity was 60 and 35% of the activity at 17 DAF, respectively. At 17 DAF, 18:1-CoA was also the best acyl donor. At 24 and 31 DAF, the best acyl donor for PC remodelling was 18:2-CoA. In all analysed stages of seed development, 18:3-CoA was the worst acyl donor. The backward activity with 18:3-CoA as a fatty acid donor declined slightly with seed development time, while there was no clear tendency in the case of 18:2-CoA (Table 4).
In the backward reactions catalysed by LPCATs, apart from LPC, an equimolar amount of acyl-CoA is created (the acyl moiety comes predominantly from the sn-2 position of PC). Thus, the capacity of this reaction (measured as described above) also indicates the capacity of the remodelling of the acyl-CoA pool available for different acyltransferases (Lager et al. 2013; Jasieniecka-Gazarkiewicz et al. 2016). Considering this relationship, we calculated the potential amount of polyunsaturated fatty acids that could be transferred from PC to the acyl-CoA pool (via the backward reactions catalysed by CsLPCATs) to make them available for other processes, e.g., TAG synthesis. In this calculation, we used the capacity of the backward reaction obtained in the assays with [14C]18:1-CoA as fatty acid donors for PC remodelling (pmol [14C]PC/nmol microsomal PC/min; Table 4), the amount of nmol PC/seed (Fig. 3) and the time between the analysed stages of seed development. For the period between 10 and 17 DAF, we took the backward reaction activity of LPCATs at 17 DAF and the average value of PC content/seed at 10 and 17 DAF (as seeds at 10 DAF were not used for microsomal preparation). For the calculations between 17 and 24 DAF and between 24 and 31 DAF, we used the average values of the LPCAT backward reaction activity and the average amount of PC/seed between these stages. We obtained the potential amount of fatty acids that could be transferred from PC to the acyl-CoA pool between the analysed stages of seed development by multiplying the average value of the backward reaction activity by the average amount of PC/seed (which indicated the amount of enzymes in the whole seed) and by the time between the analysed stages. The calculated potential transfer of fatty acids from PC to the acyl-CoA pool via the backward reactions of CsLPCATs was high and ranged from approximately 100% (early stages of development) to approximately a half (later stages of development) of the total amount of fatty acids accumulated in triacylglycerols in C. sativa developing seeds (Fig. 2). The relative amount of polyunsaturated fatty acids in the TAG of mature C. sativa seeds is approximately 55%. Thus, the capacity of the backward reaction of CsLPCATs present in developing seeds should be sufficient to transfer these fatty acids from the place of synthesis (PC) to the acyl-CoA pool available for TAG biosynthesis. It should be noted, however, that these estimations represent only a theoretical possibility of fatty acid transfer from PC to the acyl-CoA pool and are based on the backward reaction capacity of CsLPCATs determined with in vitro assays.
Discussion
It has been postulated since the 1980s that LPCATs play a role in acyl exchange between PC and the acyl-CoA pool via the backward reaction (Stymne and Glad 1981; Stymne and Stobart 1984). However, the fact that LPCATs can operate in both the forward and backward directions has been definitively proven only recently (Lager et al. 2013; Pan et al. 2015; Jasieniecka-Gazarkiewicz et al. 2016). To date, the capacity of the backward reactions of LPCATs in the developing seeds of oilseed plants has not been specified; therefore, it was not possible to establish to what extent they could be responsible for the transfer of polyunsaturated fatty acids from PC to the acyl-CoA pool. In this study, we successfully determined the backward reaction capacity of LPCATs in microsomal fractions from C. sativa seeds at different developmental stages. By evaluating enzyme activity in aliquots of microsomal fractions containing 1 nmol of PC and by estimating the PC content in individual seeds at each analysed stage of development, we were able to calculate the entire enzyme activity in the seeds and consequently the amount of fatty acids that could be transferred from PC to the acyl-CoA pool throughout seed development. The obtained data clearly indicate that the CsLPCATs present in developing seeds have the potential to transfer almost all the polyunsaturated fatty acids (half of total fatty acids) synthesised in PC to the acyl-CoA pool. This statement does not imply that LPCATs outcompete other enzymes in vivo (see Introduction) that are involved in the transfer of the modified fatty acids in PC to other lipids, e.g., TAG. This finding means only that in C. sativa seeds, LPCATs could be important or major players in this process. CsLPCATs do not discriminate between polyunsaturated fatty acids and oleic acid for remodelling PC. Thus, under in vivo conditions, the relative availability of 18:1-CoA and other acyl-CoAs for CsLPCATs will determine what kind of PC is synthesised. However, as over 55% of all TAG fatty acids are polyunsaturated, 18:1 has to be efficiently incorporated into PC and undergo the desaturation process. This study did not evaluate the acyl-CoA profile of developing C. sativa seeds. However, previous studies (Ruiz-Lopez et al. 2016) have shown that the acyl-CoA pool of C. sativa seeds at 28 DAF (which roughly corresponds to a period between the second—24 DAF—and the third—31 DAF—stage of seed development in our study) contains approximately 2.6 times more 18:1-CoA than 18:2-CoA and approximately 1.5 times more 18:1-CoA than 18:3-CoA. The good utilisation of 18:2-CoA and 18:3-CoA for PC remodelling was a surprising result. At the outset, we assumed that the physiological function of this process is a transfer of polyunsaturated fatty acids from the place of biosynthesis (PC) to the acyl-CoA pool available for different lipid biosynthesis/remodelling (Scheme 3). One of the possible explanations of this phenomenon could be that the 18:1-CoA available for elongation by the cytosolic elongation system comes not only directly from plastids (the place of synthesis) but possibly from the PC pool (Bao et al. 1998). The improved utilisation of 18:2-CoA compared with 18:1-CoA for PC remodelling at 24 DAF and 31 DAF of seed development than at 17 DAF correlates well with the higher rate of biosynthesis of very long chain fatty acids observed at later stages of seed development. At 17 DAF, the total amount of VLCFA accounted for approximately 8% of the total fatty acids of seed acyl lipids, whereas at 31 DAF, it accounted for more than 25%. The capacity of the backward reaction catalysed by CsLPCATs does not correlate well with the capacity of the forward reaction. The backward reaction activity of CsLPCATs was the highest at 17 DAF, and the forward reaction activity was the highest at 24 DAF. Previously, it has also been shown that LPCAT activity in the forward reaction is not correlated with its activity in the backward reactions (Jasieniecka-Gazarkiewicz et al.2016).
Biosynthetic pathway of 18C unsaturated fatty acids and the role of acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) in the transfer of these fatty acids to the acyl-CoA pool available for lipid biosynthesis (e.g., TAG) or for remodelling and elongation by the cytosolic elongation system. Desaturation and elongation processes as well as lipid biosynthesis and remodelling take place in the endoplasmic reticulum. FAD2 fatty acid desaturase 2, FAD3 fatty acid desaturase 3, TAG triacylglycerol
Storage lipids accumulated in C. sativa seeds mainly between 17 and 31 DAF. This finding was consistent with the suggestion that up to 14–17 days from flowering, seeds mainly increase the number of their cells (“growth phase”), and only after this period does the “accumulation phase” occur (Rodriguez-Rodriguez et al. 2013). The mature seeds of C. sativa contained approximately 80% unsaturated fatty acids (18:3, 37%: 18:2, 18%; 20:1, 13%; and 18:1, 12%, approximately). Similar fatty acid compositions of acyl lipids of C. sativa seeds have also been reported by others (Zubr 1997; Gugel and Falk 2006; Rodriguez-Rodriguez et al. 2013; Marmon et al. 2017). After 31 DAF, the net increase in the acyl lipids in C. sativa seeds was not recorded. However, at that time, the relative amount of TAG increased by approximately 3%. Additionally, at that time period, the relative amount of 18:3 in the acyl lipids of C. sativa seeds (of which approximately 93% were TAG) increased by approximately 3%. At the same time, the relative amount of 18:3 in the “polar lipid” class decreased by approximately 21%. Thus, one can propose that at this stage of seed development, the synthesis of TAG occurs via the remodelling of the acyl lipids present in the seeds, and some kind of exchange of fatty acids between polar and storage lipids occurs and the desaturation process can still occur.
The LPCATs of C. sativa seeds operate with the highest activity at a pH of 9.0–10.0, i.e., under conditions that do not exist in the natural environment of these enzymes. However, this could be a common feature for the LPCATs in different organisms. For instance, the LPCATs of B. napus have the highest activity at a pH of 10.0 (Oo and Huang 1989; Furukawa-Stoffer et al. 2003), and the same is true in the case of LPCAT1 present in the lungs of mice (Nakanishi et al. 2006). CsLPCATs were inhibited by Mg2+ and Ca2+ ions and, to a certain extent, by K+ ions. In the case of magnesium and calcium, the inhibition was not linearly correlated with the tested ion concentrations. This finding could suggest that different mechanisms of inhibition occurred at low and high concentrations of these ions. At concentrations of both Mg2+ and Ca2+ as low as 0.05 mM, it could be speculated that the inhibition was directly connected to the modification of the enzyme structure caused by these ions. Such a concentration could be the saturation point for the enzyme, as even a fivefold increase in ion concentration did not further change the enzyme activity. Any further decrease in LPCAT activity could be explained by the influence of other types of tested ions. For instance, it was shown that Mg2+ ions at concentrations above 5 mM could affect acyl-CoA solubility (Constantinides and Steim 1986). The total Mg2+ concentration in cells ranges from 15 to 25 mM (Moomaw and Maguire 2008). However, most Mg2+ ions are bound or incorporated into cellular components, which leaves only approximately 0.4–0.5 mM as free cytosolic Mg2+(Karley and White 2009; Maathuis 2009). Even that concentration could already affect the acyl-CoA solubility, nevertheless, it must be noted that in cells, acyl-CoAs are bound by acyl-CoA-binding protein, which also influences solubility (Yurchenko et al. 2009). Thus, the results from in vitro studies cannot be directly implemented under in vivo conditions. The inhibition of the LPCATs in microsomal fractions of rat submandibular glands and rat heart activity by 10 mM Ca2+ and Mg2+ has previously been reported (Soupene et al. 2008). The inhibition of CsLPCATs by K+ was not as spectacular as the inhibition caused by magnesium and calcium (10 and 30% inhibition at 0.5 mM and 2 mM, respectively); however, plant cells maintain a relatively high K+ concentration (100–200 mM) in the cytosol (Blumwald et al. 2000). Thus, in nature, potassium ions could have some effects on LPCAT activity.
The positional specificity of the CsLPCATs of seed microsomal fractions does not differ much from that of Arabidopsis LPCATs. Lager et al. (2013), in experiments similar to those performed in this study, proved that AtLPCAT1 and AtLPCAT2 show approximately 7–8 times higher activity towards the sn-2 position than towards the sn-1 position of LPC when 18:1-CoA is the fatty acid donor and approximately 3–5 times higher in cases when 16:0-CoA is the fatty acid donor. Thus, both studies indicate that LPCATs preferentially acylate the sn-2 position of LPC and can acylate the sn-1 position, with much lower efficiency. The LPCATs in the microsomal fractions of C. sativa seeds showed a clear preference for 18C unsaturated acyl-CoA in the forward reaction. Similar substrate specificity of the LPCATs in the microsomal fractions of yeast with mutated main endogenous acyl-CoA:lysophospholipid acyltransferase (ALE1) overexpressed with genes encoding the LPCATs of A. thaliana, L. fendleri, C. tinctorius, R. communis, H. benghalensis and L. usitatissimum were previously shown (Lager et al. 2013; Pan et al. 2015). Thus, it seems that the LPCATs in oilseed plants do not discriminate between 18:2-CoA and 18:3-CoA in favour of 18:1-CoA. This should be expected, as 18:1 undergoes a desaturation process after incorporation into PC. Thus, similar to our conclusions on the utilisation of acyl-CoA for PC remodelling connected with the backward reaction of LPCATs, the availability of 18:1-CoA (which is, according to Ruiz-Lopez et al. 2016, higher than that of polyunsaturated acyl-CoA), 18:2-CoA and 18:3-CoA most likely decides what kind of PC is synthesised via LPCAT action. The preferences of CsLPCAT for different acyl-CoAs obtained in assays with single acyl-CoA were confirmed in substrate selectivity assays where five different acyl-CoAs were added to the reaction mixtures in equimolar concentrations. However, under in vivo conditions, the presence of acyl-CoA binding proteins (ACBPs) could affect the preferences of LPCATs for different acyl-CoAs. Thus, in one variation of the substrate selectivity assays, we added BSA to the reaction buffer (in in vitro assays, the ACBPs are often replaced by BSA) at a concentration of 2 mg/ml. The concentration of BSA used was probably in excess of the concentration of ACBPs present in the cells. Engeseth et al. (1996) showed, for example, that in the tissues of A. thaliana, the concentrations of ACBPs ranged between 3 and 143 µg/g FW. The obtained results were quite similar to those obtained in the experiments without the addition of BSA (only a slight modification of the preferences of CsLPCATs towards different acyl-CoA were noted). This finding indicates that the preferences of CsLPCATs towards different acyl-CoA measured in our in vitro assays could also be similar to those under in vivo conditions. According to Ruiz-Lopez et al. (2016), a substantial amount of the cytosolic acyl-CoA pool consists of very long chain fatty acid-CoAs. For instance, 20:1-CoA constitutes approximately 19.5% and 22:1-CoA constitutes approximately 10% of this pool. However, these fatty acids are observable only in trace amounts in PC (data not presented). This result correlates well with the very low affinity of CsLPCATs for these acyl-CoAs. Thus, contrary to 18C-acyl-CoA, the substrate specificity of CsLPCATs determines the presence of these fatty acids in PC only in trace amounts.
The activity of CsLPCAT was the highest at 24 DAF; however, it was also quite high at 17 DAF and 31 DAF. The observed LPCAT activity was probably generated by different isoforms of CsLPCATs. The data in the genome database of C. sativa indicate the presence of six CsLPCAT isoforms. Three of these isoforms probably encode LPCAT1, and the other three probably encode LPCAT2. However, this assumption was made only based on the similarity of the genes encoding these isoforms to the genes encoding the LPCATs of A. thaliana. To date, no experimental data have confirmed that these genes truly encode CsLPCATs. Preliminary data on the expression levels of these candidate genes of CsLPCATs were presented by Abdullah et al. (2016). This data showed that between the first stage of seed development (10–15 DAF) and the second stage (16–21 DAF), the expression levels of all candidate genes encoding CsLPCAT isoforms increased. This finding is in agreement with our results showing an increase in CsLPCAT activity between these stages of seed development. The acylation rate of LPC to form PC in our assays using the crude microsomal fraction could be a result of not only CsLPCAT activity but also of CsLPEAT (acyl-CoA:lysophosphatidylethanolamine acyltransferases) activity, as it was shown earlier that LPEAT enzymes can also acylate LPC to some degree (Jasieniecka-Gazarkiewicz et al. 2016). In our preliminary assays, we have shown, however, that the activity of CsLPEAT in seed microsomal fractions accounted for only approximately 10% of the activity of CsLPCAT. Thus, the effects of endogenous CsLPEATs on the obtained results concerning the activity and substrate specificity of CsLPCATs seem to be negligible.
Author contribution statement
SK, KJ-G and AB conceived and designed the research. SK and KJ-G conducted the experiments. SK, KJ-G and AB analysed the data and wrote the manuscript. All authors read and approved the manuscript.
Abbreviations
- DAF:
-
Days after flowering
- DTNB:
-
Dithionitrobenzoic acid
- FA:
-
Fatty acid
- LPC:
-
Lysophosphatidylcholine
- LPCAT:
-
Acyl-CoA:lysophosphatidylcholine acyltransferase
- LPLAT:
-
Acyl-CoA:lysophospholipid acyltransferase
- PC:
-
Phosphatidylcholine
- TAG:
-
Triacylglycerol
- VLCFA:
-
Very long chain fatty acids
- 10:0:
-
Capric acid
- 12:0:
-
Lauric acid
- 14:0:
-
Myristic acid
- 16:0:
-
Palmitic acid
- 18:0:
-
Stearic acid
- 18:1:
-
Oleic acid
- 18:2:
-
Linoleic acid
- 18:3:
-
α-Linolenic acid
- 20:1:
-
Gondoic acid
- 22:1:
-
Erucic acid
References
Abdullah HM, Akbari P, Paulose B, Schnell D, Qi W, Park Y, Pareek A, Dhankher OP (2016) Transcriptome profiling of Camelina sativa to identify genes involved in triacylglycerol biosynthesis and accumulation in the developing seeds. Biotechnol Biofuels 9:136
Banaś A, Dahlqvist A, Ståhl U, Lenman M, Stymne S (2000) The involvement of phospholipid:diacylglycerol acyltransferases in triacylglycerol production. Biochem Soc Trans 28:703–705
Bao X, Pollard M, Ohlrogge J (1998) The biosynthesis of erucic acid in developing embryos of Brassica rapa. Plant Physiol 118(1):183–190
Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C (2012) Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol 160:1530–1539
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Blumwald E, Aharon G, Apse M (2000) Sodium transport in plant cells. Biochem Biophys Acta 1465(1–2):140–151
Browse J, Somerville C (1991) Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol 42:467–506
Constantinides PP, Steim JM (1986) Solubility of palmitoyl-coenzyme A in acyltransferase assay buffers containing magnesium ions. Arch Biochem Biophys 250:267–270
Dahlqvist A, Ståhl U, Lenman M, Banaś A, Lee M, Sandager L, Ronne H, Stymne S (2000) Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97:6487–6492
Engeseth NJ, Pacovskya RS, Newman T, Ohlrogge JB (1996) Characterization of an acyl-CoA-binding protein from Arabidopsis thaliana. Arch Biochem Biophys 331(1):55–62
Fraser T, Stobart K (2000) Partial purification and photoaffinity labelling of sunflower acyl-CoA:lysophosphatidylcholine acyltransferase. Biochem Soc Trans 28(6):715–718
Furukawa-Stoffer TL, Boyl RM, Thomson AL, Sarna MA, Weselake RJ (2003) Properties of lysophosphatidylcholine acyltransferase from Brassica napus cultures. Lipids 38:651–656
Gugel RK, Falk KC (2006) Agronomic and seed quality evaluation of Camelina sativa in western Canada. Can J Plant Sci 86:1047–1058
Ichihara K, Mae K, Sano Y, Tanaka K (1995) 1-Acyl-glycerophosphocholine O-acyltransferase in maturing safflower seeds. Planta 196:551–557
Jasieniecka-Gazarkiewicz K, Demski K, Lager I, Stymne S, Banaś A (2016) Possible role of different yeast and plant lysophospholipid:acyl-CoA acyltransferases (LPLATs) in acyl remodelling of phospholipids. Lipids 51:15–23
Karley AJ, White PJ (2009) Moving cationic minerals to edible tissues: potassium, magnesium, calcium. Curr Opin Plant Biol 12:291–298
Kennedy EP (1961) Biosynthesis of complex lipids. Fed Proc 20:934–940
Lager I, Yilmaz JL, Zhou XR, Jasieniecka K, Kazachkov M, Wang P, Zou J, Weselake R, Smith MA, Bayon S et al (2013) Plant acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) have different specificities in their forward and reverse reactions. J Biol Chem 288(52):36902–36914
Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Debono A, Durrett TP et al (2013) Acyl-lipid metabolism. Arabidopsis Book 11:e0161
Maathuis FJM (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12:250–258
Marmon S, Sturtevant D, Herrfurth C, Chapman K, Stymne S, Feussner I (2017) Two acyltransferases contribute differently to linolenic acid levels in seed oil. Plant Physiol 173:2081–2095
Moomaw AS, Maguire ME (2008) The unique nature of Mg2+ channels. Physiology 23:275–285
Nakanishi H, Shindou H, Hishikawa D, Harayama T, Ogasawara R, Suwabe A, Taguchi R, Shimizu T (2006) Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1) expression in alveolar type ii cells and possible involvement in surfactant production. J Biol Chem 281:20140–20147
Oo KC, Huang AH (1989) Lysophosphatidate acyltransferase activities in the microsomes from palm endosperm, maize scutellum, and rapeseed cotyledon of maturing seeds. Plant Physiol 91:1288–1295
Pan X, Chen G, Kazachkov M, Greer MS, Caldo KM, Zou J, Weselake RJ (2015) In vivo and in vitro evidence for biochemical coupling of reactions catalyzed by lysophosphatidylcholine acyltransferase and diacylglycerol acyltransferase. J Biol Chem 290:18068–18078
Putnam DH, Budin JT, Field LA, Breene WM (1993) Camelina: a promising low-input oilseed. In: Janick J, Simon JE (eds) New crops. Wiley, New York, pp 314–322
Rodriguez-Rodriguez MF, Sanchez-Garcia A, Salas JJ, Garces R, Martínez-Force E (2013) Characterization of the morphological changes and fatty acid profile of developing Camelina sativa seeds. Ind Crops Prod 50:673–679
Ruiz-Lopez Broughton R, Usher S, Salas JJ, Haslam RP, Napier JA, Beaudoin F (2016) Tailoring the composition of novel wax esters in the seeds of transgenic Camelina sativa through systematic metabolic engineering. Plant Biotechnol J 15:837–849
Sánchez M, David GN, David NB (1973) The relationship between palmitoyl-coenzyme A synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria. Biochem J 132:697–706
Shindou H, Hishikawa D, Harayama T, Yuki K, Shimizu T (2009) Recent progress on acyl CoA:lysophospholipid acyltransferase research. J Lipid Res 50:46–51
Slack CR, Campbell LC, Browse JA, Roughan PG (1983) Some evidence for the reversibility of the cholinephosphotransferase-catalysed reaction in developing linseed cotyledons in vivo. Biochem Biophys Acta 754:10–20
Soupene E, Fyrst H, Kuypers FA (2008) Mammalian acyl-CoA:lysophosphatidylcholine acyltransferase enzymes. Proc Natl Acad Sci USA 105(1):88–93
Ståhl U, Stålberg K, Stymne S, Ronne H (2008) A family of eukaryotic lysophospholipid acyltransferases with broad specificity. FEBS Lett 582:305–309
Stymne S, Glad G (1981) Acyl exchange between oleoyl-CoA and phosphatidylcholine in microsomes of developing soya bean cotyledons and its role in fatty acid desaturation. Lipids 16:298–305
Stymne S, Stobart AK (1984) Evidence for the reversibility of the acyl-CoA:lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem J 223:305–314
Tumaney AW, Rajasekharan R (1999) Synthesis of azidophospholipids and labeling of lysophosphatidylcholine acyltransferase from developing soybean cotyledons. Biochem Biophys Acta 1439:47–56
Yurchenko OP, Nykiforuk CL, Moloney MM, Ståhl U, Banaś A, Stymne S, Weselake RJ (2009) A 10-kDa acyl-CoA-binding protein (ACBP) from Brassica napus enhances acyl exchange between acyl-CoA and phosphatidylcholine. Plant Biotechnol J 7:602–610
Zhang D, Jasieniecka-Gazarkiewicz K, Wan X, Luo L, Zhang Y, Banaś A, Jiang M, Gong Y (2015) Molecular characterization of two lysophospholipid:acyl-CoA acyltransferases belonging to the MBOAT family in Nicotiana benthamiana. PLoS One 10(12):e0144653
Zheng Q, Li JQ, Kazachkov M, Liu K, Zou J (2012) Identification of Brassica napus lysophosphatidylcholine acyltransferase genes through yeast functional screening. Phytochemistry 75:21–31
Zubr J (1997) Oil-seed crop: Camelina sativa. Ind Crops Prod 6:113–119
Funding
The project was partially financed by the National Science Centre (NCN), Poland. Project number: UMO-2017/25/B/NZ3/00721.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Klińska, S., Jasieniecka-Gazarkiewicz, K. & Banaś, A. Acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) of Camelina sativa seeds: biochemical properties and function. Planta 250, 1655–1670 (2019). https://doi.org/10.1007/s00425-019-03248-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-019-03248-6