Abstract
Main conclusion
Verticillium colonization does induce a cascade of defense/stress proteins but the Ve1 gene also promotes enhanced root growth, which appears to allow the plant to outgrow the pathogen and avoid symptoms associated with an exaggerated defense response.
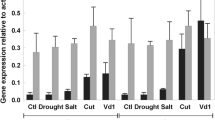
In tomato, the Ve1 gene provides resistance to the vascular pathogen, Verticillium dahliae, race 1; ve1 plants are susceptible. However, the physiological basis of the resistance is unknown. While developing alternative lines of mutant Ve1 gene transformants to address this question a striking difference was observed in transformation frequency resulting from the inefficient rooting of plantlets from ve1 callus relative to Ve1 callus. Subsequent experiments with resistant and susceptible near-isolines of the cultivar Craigella, as well as Ve1 transformants, showed that in both artificial medium and soil, root growth was significantly enhanced in the resistant cultivar. Parallel studies of Verticillium colonization indicated a significantly lower overall concentration in the resistant plant characteristic of the resistant phenotype, but an almost equal total fungal biomass in both resistant and susceptible roots. Proteomic analyses of the roots of Verticillium-infected plants revealed elevated levels of defense/stress proteins, which correlated with the fungal concentration rather than resistance. Hormone analyses demonstrated a higher cis-ABA level in the resistant isoline consistent with enhanced root growth. Taken together these studies indicate a similar fungal biomass in the roots of both isolines where the Ve1 gene also promotes root production. In the case of the Craigella/Vd1 pathosystem, this appears to allow the host to resist better by outgrowing the pathogen with less wilt rather than reliance only on partial immunity.






Similar content being viewed by others
Abbreviations
- CR:
-
Craigella-resistant mutant
- CS:
-
Craigella-susceptible mutant
References
Beckman CH (1987) The nature of wilt diseases of plants. APS Press, St. Paul
Bishop CD, Cooper RM (1983a) An ultrastructural study of vascular colonization in three vascular wilt diseases. I Colonization of susceptible cultivars. Physiol Plant Pathol 23:323–343
Bishop CD, Cooper RM (1983b) An ultrastructure study of root invasion in three vascular wilt diseases. Physiol Plant Pathol 22:15–27
Bishop CD, Cooper RM (1984) Ultrastructure of vascular colonization by fungal wilt pathogens. II. Invasion of resistant cultivars. Physiol Plant Pathol 24:277–289
Busch LV, Smith E (1981) Susceptibility of Ontario-grown alflafa cultivars and certain Medicago species to Verticillium albo-atrum. Can J Plant Pathol 3:169–172
Castroverde CDM (2016) Molecular biology of the tomato Ve gene family. PhD molecular and cellular biology. University of Guelph, Guelph, pp 1–205
Castroverde CDM, Xu X, Blaya Fernandez J, Nazar RN, Robb J (2017) Epistatic influence in tomato ve1-mediated resistance. Plant Biol 19:843–847
Chen P, Lee B, Robb J (2004) Tolerance to a non-host isolate of Verticillium dahliae in tomato. Physiol Mol Plant Pathol 64:283–291
Chiwocha SDS, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross A, Kermode AR (2003) A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrosprayionization tandem mass spectrometry: analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J 35:405–417
Chiwocha SDS, Cutler AJ, Abrams SR, Ambrose SJ, Yang Y, Ross AR, Kermode AR. (2005) The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J 42:35–48
de Jonge R, van Esse HP, Maruthachalam K, Bolton MD, Santhanam P, Saber MK, Zhang Z, Usami T, Lievens B, Subbarao KV, Thomma BPHJ (2012) Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc Natl Acad Sci USA 109:5110–5115
Develey-Riviere M-P, Galiana E (2007) Resistance to pathogens and host developmental stage: a multifaceted relationship within the plant kingdom. New Phytol 175:405–416
Diwan N, Fluhr R, Eshed Y, Zamir D, Tanksley SD (1999) Mapping of Ve in tomato: a gene conferring resistance to the broad-spectrum pathogen, Verticillium dahliae Kleb. race 1. Theor Appl Genet 98:315–319
Dobinson KF, Grant SJ, Kang S (2004) Cloning and targeted disruption, via Agrobacterium tumefaciens-mediated transformation, of a trypsin protease gene from the vascular wilt fungus Verticillium dahliae. Curr Genet 45:104–110
Elgersma DM, Liem JI (1989) Accumulation of phytoalexins in susceptible and resistant near-isogenic lines of tomato infected with Verticillium albo-atrum or Fusarium oxysporum f.sp. lycopersici. Physiol Mol Plant Pathol 34:545–555
Flor HH (1942) Inheritance of pathogenicity in Melampsora lini. Phytopathology 32:653–669
Flor HH (1971) Current status of the gene-for-gene concept. Annu Rev Phytopathol 9:275–296
Fradin EF, Thomma BPHJ (2006) Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol Plant Pathol 7:71–86
Fradin EF, Zhang Z, Juarez Ayala JC, Castroverde CDM, Nazar RN, Robb J, Liu C-M, Thomma BPHJ (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol 150:320–332
Fradin EF, Abd-El-Haliem A, Masini L, van den Berg GCM, Joosten MHAJ, Thomma BPHJ (2011) Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis. Plant Physiol 156:2255–2265
Fradin EF, Zhang Z, Rovenich H, Song Y, Liebrand TW, Masini L, van den Berg GC, Joosten MH, Thomma BPHJ (2014) Functional analysis of the tomato immune receptor Ve1 through domain swaps with its non-functional homologue Ve2. PLoS ONE 9:e88208
Gold J, Robb J (1995) The role of the coating response in Craigella tomatoes infected with Verticillium dahliae, races 1 and 2. Physiol Mol Plant Pathol 47:141–157
Harris JM (2015) Abscisic acid: hidden architect of the root system structure. Plants (Basel) 4:548–572
Harrison SJ, Mott EK, Parsley K, Aspinall S, Gray JC, Cottage A (2006) A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods 2:19
Hoagland DR, Arnon D (1950) The water culture method of growing plants without soil (California Agricultual Experiment Station), p 347
Holt BFI, Boyes DC, Ellerstrom M, Siefers N, Wiig A, Kauffman S, Grant MR, Dangl JL (2002) An evolutionarily conserved mediator of plant disease resistance gene function is required for normal Arabidopsis development. Dev Cell 2:807–817
Hu X, Nazar RN, Robb J (1993) Quantification of Verticillium biomass in wilt disease development. Physiol Mol Plant Pathol 42:23–36
Hurkman WJ, Tanaka CK (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81:802–806
Hutson RA, Smith IM (1980) Phytoalexins and tyloses in tomato cultivars infected with Fusarium oxysporum f.sp. lycopersici or Verticillium albo-atrum. Physiol Plant Pathol 17:245–257
Inderbitzin P, Bostock RM, Davis RM, Usami T, Platt HW, Subbarao KV (2011) Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS ONE 6(12):e28341
Jones JDG, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kawchuk L, Hachey J, Lynch DR, Klcsar F, van Rooijen G, Waterer DR, Robertson A, Kokko E, Byers R, Howard RJ, Fischer R, Prufer D (2001) Tomato Ve disease resistance genes encode cell surface-like receptors. Proc Natl Acad Sci USA 98:6511–6515
Liebrand TWH, van den Berg GCM, Zhang Z, Smit P, Cordewener JHG, America AHP, Sklenar J, Jones AME, Tameling WIL, Robatzek S, Thomma BPHJ, Joosten MHAJ (2013) Receptor-like kinase SOBIR1/EVR interacts with receptor-like proteins in plant immunity against fungal infection. Proc Natl Acad Sci USA 110:10010–10015
Mace ME, Bell AA, Beckman CH (1981) Fungal wilt diseases of plants. Academic Press, New York
McAdam SAM, Brodribb TJ, Ross JJ (2016) Shoot-drrived abscisic acid promotes root growth. Plant Cell Environ 39:652–659
McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R, Fraley R (1986) Leaf disc transformation of cultivated tomato (L.esculentum) using Agrobacterium tumefaciens. Plant Cell Rep 5:81–84
Pegg GF (1976) The response of ethylene treated tomato plants to infection by Verticillium albo-atrum. Physiol Plant Pathol 9:215–226
Pegg GF, Brady BL (2002) Verticillium wilts. CABI Publishing, Wallingford
Pegg GF, Street PFS (1984) Measurement of Verticillium albo-atrum in high and low resistance hop cultivars. Trans Br Mycol Soc 82:99–106
Petit-Houdenot Y, Fudal I (2017) Complex interactions between fungal avirulence genes and their corresponding plant resistance genes and consequences for plant disease management. Front Plant Sci. https://doi.org/10.3399/fpls.2017.01072
Pierret A, Moran CJ, Doussan C (2005) Conventional detection methodology is limiting our ability to understand the roles and functions of fine roots. New Phytol 166:967–980
Robb EJ, Nazar RN (1996) Factors contributing to successful PCR-based diagnostics for potato and other crops. In: Marshall G (ed) Diagnostics in crop production. British Crop Protection Council, Surrey, pp 75–84
Robb J, Powell DA, Street PFS (1987) Time course of wall coating secretion in Verticillium-infected tomatoes. Physiol Mol Plant Pathol 31:217–226
Robb EJ, Shittu HO, Soman KV, Kurosky A, Nazar RN (2012) Elevated defense protein fails to protect tomato against Verticillium dahliae. Planta 236:623–633
Ross ARS, Ambrose SJ, Cutler AJ, Feurtado JA, Kermode AR, Nelson K, Zhou R, Abrams SR (2004) Determination of endogenous and supplied deuterated abscisic acid in plant tissues by high performance liquid chromatography-electrospray ionization tandem mass spectrometry with multiple reaction monitoring. Anal Biochem 329:324–333
Schaible L, Cannon OS, Waddoups V (1951) Inheritance of resistance to Verticillium wilt in a tomato cross. Phytopathology 41:986–990
Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25:211–222
Shittu HO, Castroverde CDM, Nazar RN, Robb J (2009) Plant-endophyte interplay protects tomato against a virulent Verticillium. Planta 229:415–426
Song Y, Zhang Z, Seidl MF, Majer A, Jakse J, Javornik B, Thomma BP (2017) Broad taxonomic characterization of Verticillium wilt resistance genes reveals an ancient origin of the tomato Ve1 immune receptor. Mol Plant Pathol 18(2):195–209
Street PFS, Robb J, Ellis BE (1986) Secretion of vascular coating components by xylem parenchyma cells of tomatoes infected with Verticillium albo-atrum. Protoplasma 132:1–11
Subedi KD, Ma BL, Liang BC (2006) New method to estimate root biomass in soil through root-derived carbon. Soil Biol Biochem 38:2212–2218
Thomma BP, Nürnberger T, Joosten MH (2011) Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23(1):4–15
Van Ooijin G, Van der Burg HA, Cornelissen BJC, Takken FLW (2007) Structure and function of resistance proteins in Solanaceous plants. Annu Rev Phytopathol 45:43–72
Warnock SJ (1991) Natural habitats of Lycopersicon species. HortScience 26:466–471
Xu X, Robb J, Nazar RN (2017) A “whole pot” strategy for root growth quantification or microbiota-root interaction studies. Soil Biol Biochem 111:154–156
Zhang Z, van Esse HP, van Damme M, Fradin EF, Liu CM, Thomma BPHJ (2013) Ve1-mediated resistance against Verticillium does not involve a hypersensitive response in Arabidopsis. Mol Plant Pathol 14:719–727
Zhang Z, Song Y, Liu CM, Thomma B (2014) Mutational analysis of the Ve1 immune receptor that mediates Verticillium resistance in tomato. PLoS ONE 9:e99511
Acknowledgements
We thank Drs. X. Luo and K. V. Soman (UTMB Proteomics Center) for the peptide mass spectrometry and help with the proteomic analyses and Dr. Irina Zaharia (National Research Council of Canada) for the plant hormone analyses. This study was supported by NSERC (R. N. N. and J. R.) and NIH, NHLBI (A. K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nazar, R.N., Xu, X., Shittu, H. et al. Tomato Ve resistance locus; defense or growth. Planta 247, 1339–1350 (2018). https://doi.org/10.1007/s00425-018-2869-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-2869-y