Abstract
Main conclusion
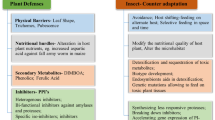
Multiplicity of protease inhibitors induced by predators may increase the understanding of a plant’s intelligent behavior toward environmental challenges.
Information about defense mechanisms of non-genomic model plant passion fruit (Passiflora edulis Sims) in response to predator attack is still limited. Here, via biochemical approaches, we showed its flexibility to build-up a broad repertoire of potent Kunitz-type trypsin inhibitors (KTIs) in response to methyl jasmonate. Seven inhibitors (20–25 kDa) were purified from exposed leaves by chromatographic techniques. Interestingly, the KTIs possessed truncated Kunitz motif in their N-terminus and some of them also presented non-consensus residues. Gelatin-Native-PAGE established multiple isoforms for each inhibitor. Significant differences regarding inhibitors’ activity toward trypsin and chymotrypsin were observed, indicating functional polymorphism. Despite its rarity, two of them also inhibited papain, and such bifunctionality suggests a recruiting process onto another mechanistic class of target protease (cysteine-type). All inhibitors acted strongly on midgut proteases from sugarcane borer, Diatraea saccharalis (a lepidopteran insect) while in vivo assays supported their insecticide properties. Moreover, the bifunctional inhibitors displayed activity toward midgut proteases from cowpea weevil, Callosobruchus maculatus (a coleopteran insect). Unexpectedly, all inhibitors were highly effective against midgut proteases from Aedes aegypti a dipteran insect (vector of neglected tropical diseases) opening new avenues for plant-derived PIs for vector control-oriented research. Our results reflect the KTIs’ complexities in passion fruit which could be wisely exploited by influencing plant defense conditions. Therefore, the potential of passion fruit as source of bioactive compounds with diversified biotechnological application was strengthened.







Similar content being viewed by others
Abbreviations
- BANA:
-
N-Benzoyl-l-arginine-2-naphthylamide
- BAPNA:
-
N-Benzoyl-dl-arginyl-plnitroanilide
- BSA:
-
Bovine serum albumin
- BTEE:
-
N-Benzoyl tyrosine ethyl ester
- eggCys:
-
Egg cystatin
- EST:
-
Expressed sequence tags
- KPIs:
-
Kunitz-type protease inhibitors
- KTI:
-
Kunitz-type trypsin inhibitors
- MeJa:
-
Methyl jasmonate
- NCBI:
-
National Center for Biotechnology Information
- PIs:
-
Proteinase inhibitors
- SDS:
-
Sodium dodecyl sulfate
- SoyKTI:
-
Soybean Kunitz inhibitor
- TFA:
-
Trifluoroacetic acid
- TIs:
-
Trypsin inhibitors
References
Abe M, Abe K, Iwabucni K, Domoto C, Arai S (1994) Corn cystatin I expressed in Escherichia coli: investigation of its inhibitory profile and occurrence in corn kernels. J Biochem 116:488–492
Azarkan M, Martinez-Rodriguez S, Buts L, Baeyens-Volant D, Garcia-Pino A (2011) The plasticity of the trefoil fold constitutes an evolutionary platform for protease inhibition. J Biol Chem 286:43726–43734
Bhattacharyya A, Babu CR (2009) Purification and biochemical characterization of a serine proteinase inhibitor from Derris trifoliata Lour. seeds: insight into structural and antimalarial features. Phytochemistry 70:703–712
Bhattacharyya A, Mazumdar S, Leighton SM, Babu CR (2006) A Kunitz proteinase inhibitor from Archidendron ellipticum seeds: purification, characterization, and kinetic properties. Phytochemistry 67:232–241
Botelho-Júnior S, Siqueira-Júnior CL, Jardim BC, Machado OLT, Neves-Ferreira AGC, Perales J, Jacinto T (2008) Trypsin inhibitors in passion fruit (Passiflora f. edulis flavicarpa) leaves: accumulation in response to methyl jasmonate, mechanical wounding, and herbivory. J Agric Food Chem 56:9404–9409
Campos FAP, Xavier-Filho J, Silva CP, Ary MB (1989) Resolution and partial characterization of proteinases and α-amylases from midguts of larvae of the bruchid beetle Callosobruchus maculatus (F.). Comp Biochem Physiol 92B:51–57
Carrillo L, Martinez M, Ramessar K, Cambra I, Castañera P, Ortego F, Diaz I (2011) Expression of a barley cystatin gene in maize enhances resistance against phytophagous mites by altering their cysteine-proteases. Plant Cell Rep 30:101–112
Chandrasekaran V, Lee CJ, Lin P, Duke RE, Pedersen LG (2009) A computational modeling and molecular dynamics study of the Michaelis complex of human protein Z-dependent protease inhibitor (ZPI) and factor Xa (FXa). J Mol Model 15:897–911
Chaudhary NS, Shee C, Islam A, Ahmad F, Yernool D, Kumar P, Sharma AK (2008) Purification and characterization of a trypsin inhibitor from Putranjiva roxburghii seeds. Phytochemistry 69:2120–2126
Christeller JT (2005) Evolutionary mechanisms acting on proteinase inhibitor variability. FEBS J 272:5710–5722
Cutri L, Dornelas MC (2012) PASSIOMA: exploring expressed sequence tags during flower development in Passiflora spp. Comp Funct Genomics 2012:1–11
De Leo F, Volpicella M, Licciulli F, Liuni S, Gallerani R, Ceci LR (2002) PLANT-PIs: a database for plant protease inhibitors and their genes. Nucleic Acids Res 30:347–348
Felicioli R, Garzelli B, Vaccari L, Melfi D, Balestreri E (1997) Activity staining of protein inhibitors of proteases on gelatin containing polyacrylamide gel electrophoresis. Anal Biochem 244:176–178
Franco OL, Sá MFG, Sales MP, Mello LV, Oliveira AS, Rigden DJ (2002) Overlapping binding sites for trypsin and papain on a Kunitz-type proteinase inhibitor from Prosopis juliflora. Proteins 49:335–341
Furtado AT, Scandiffio MIG, Cortez LAB (2011) The Brazilian sugarcane innovation system. Energy Policy 39:156–166
Gáspári Z, Várnai P, Szappanos B, Perczel A (2010) Reconciling the lock-and-key and dynamic views of canonical serine protease inhibitor action. FEBS Lett 584:203–206
Goodwin PH, Xie W, Valliani M (2012) Three genes of miraculin-like proteins from Nicotiana benthamiana with dissimilar putative structures show highly similar patterns of induction following bacterial and fungal infections. Eur J Plant Pathol 134:795–810
Heady AJ, MacAskill UK, Wright MA, Claridge JK, Edwards PJB, Farley PC, Christeller JT, Laing WA, Pascal SM (2010) Solution structure of the squash aspartic acid proteinase inhibitor (SQAPI) and mutational analysis of pepsin inhibition. J Biol Chem 285:27019–27025
Heibges A, Glaczinski H, Ballvora A, Salamini F, Gebhardt C (2003a) Structural diversity and organization of three gene families for Kunitz-type enzyme inhibitors from potato tubers (Solanum tuberosum L.). Mol Genet Genomics 269:526–534
Heibges A, Salamini F, Gebhardt C (2003b) Functional comparison of homologous members of three groups of Kunitz-type enzyme inhibitors from potato tubers (Solanum tuberosum L.). Mol Genet Genomics 269:535–541
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66
Jiménez-Durán K, McClure B, Gárcia-Campusano F, Rodríguez-Sotres R, Cisneros J, Busot G, Cruz-Garcia F (2013) NaStEP: a proteinase inhibitor essential to self-incompatibility and a positive regulator of HT-B stability in Nicotiana alata pollen tubes. Plant Physiol 161:97–107
Krizaj I, Drobnic-KoSorok M, Brzin J, Jerala R, Turk V (1993) The primary structure of inhibitor of cysteine proteinases from potato. FEBS Lett 333:15–20
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laskowski M, Qasim MA (2000) What can the structures of enzyme-inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochim Biophys Acta 1477:324–337
Lemos FJA, Cornel AJ, Jacobs-Lorena M (1996) Trypsin and aminopeptidase gene expression is affected by age and food composition in Anopheles gambiae. Insect Biochem Mol Biol 26:651–658
Ma Y, Zhao Q, Lu M, Wang J (2011) Kunitz-type trypsin inhibitor gene family in Arabidopsis and Populus trichocarpa and its expression response to wounding and herbivore in Populus nigra. Tree Genet Genomes 7:431–441
Macedo ML, Garcia VA, Freire MG, Richardson M (2007) Characterization of a Kunitz trypsin inhibitor with a single disulfite bridge from seeds of Inga laurina (SW.) Wild. Phytochemistry 68:1104–1111
Major IT, Constabel CP (2008) Functional analysis of the Kunitz trypsin inhibitor family in poplar reveals biochemical diversity and multiplicity in defense against herbivores. Plant Physiol 146:888–903
Martinez M, Diaz I (2008) The origin and evolution of plant cystatins and their target cysteine proteinases indicate a complex functional relationship. BMC Evol Biol 8:198
Migliolo L, Oliveira AS, Santos EA, Franco OL, Sales MP (2010) Structural and mechanistic insights into a novel non-competitive Kunitz trypsin inhibitor from Adenanthera pavonina L. seeds with double activity toward serine and cysteine-proteinases. J Mol Graph Model 29:148–156
Mosolov VV, Valueva TA (2005) Proteinase inhibitors and their function in plants: a review. Appl Biochem Microbiol 41:227–246
Muricken DG, Gowda LR (2011) Molecular engineering of a small trypsin inhibitor based on the binding loop of horsegram seed Bowman–Birk inhibitor. J Enzyme Inhib Med Chem 26:553–560
Neuteboom LW, Matsumoto KO, Christopher DA (2009) An extended AE-rich N-terminal trunk in secreted pineapple cystatin enhances inhibition of fruit bromelain and is post translationally removed during ripening. Plant Physiol 151:515–527
Oliva ML, Silva MC, Sallai RC, Brito MV, Sampaio MU (2010) A novel subclassification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie 92:1667–1673
Oliveira RC, Docê RC, Barros STD (2012) Clarification of passion fruit juice by microfiltration: analyses of operating parameters, study of membrane fouling and juice quality. J Food Eng 111:432–439
Otlewski J, Jelen F, Zakrzewska M, Oleksy A (2005) The many faces of protease-protein inhibitor interaction. EMBO J 24:1303–1310
Patil DN, Chaudhary A, Sharma AK, Tomar S, Kumar P (2012) Structural basis for dual inhibitory role of tamarind Kunitz inhibitor (TKI) against factor Xa and trypsin. FEBS J 279:4547–4564
Pereira KRB, Botelho-Júnior S, Domingues DP, Machado OLT, Oliveira AEA, Fernandes KVS, Madureira HC, Pereira TNS, Jacinto T (2011) Passion fruit flowers: kunitz trypsin inhibitors and cystatin differentially accumulate in developing buds and floral tissues. Phytochemistry 72:1955–1961
Philippe RN, Ralph SG, Kulheim C, Jancsik SI, Bohlmann J (2009) Poplar defense against insects: genome analysis, full-length cDNA cloning, and transcriptome and protein analysis of the poplar Kunitz-type protease inhibitor family. New Phytol 184:865–884
Ramos VS, Cabrera OG, Camargo ELO, Ambrósio AB, Vidal RO, Silva DS, Guimarães LC, Marangoni SM, Parra JRP, Pereira GAG, Macedo MLR (2012) Molecular cloning and insecticidal effect of Inga laurina trypsin inhibitor on Diatraea saccharalis and Heliothis virescens. Comp Biochem Physiol C Toxicol Pharmacol 156:148–158
Rawlings ND, Waller M, Barrett AJ, Bateman A (2014) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 42:D503–D509
Ritonja A, Krizaj I, Mesko P, Kopitar M, Lucovnik P, Strukelj B, Pungercar J, Buttle DJ, Barrett AJ, Turk V (1990) The amino acid sequence of a novel inhibitor of cathepsin D from potato. FEBS Lett 267:13–15
Ryan CA (1990) Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Phytopathol 28:425–449
Selvakumar P, Gahloth D, Tomar PPS, Sharma N, Kumar Sharma AK (2011) Molecular evolution of miraculin-like proteins in soybean Kunitz super-family. J Mol Evol 73:369–379
Soares TS, Watanabe RMO, Lemos FJA, Tanaka AS (2011) Molecular characterization of genes encoding trypsin-like enzymes from Aedes aegypti larvae and identification of digestive enzymes. Gene 489:70–75
Sokal RR, Rohlf FJ (2011) Biometry, 4th edn. W.H. Freeman & Co, New York
Speranskaya AS, Krinitsina AA, Kudryavtseva AV, Poltronieri P, Santino A, Oparina NY, Dmitriev AA, Belenikin MS, Guseva MA, Shevelev AB (2012) Impact of recombination on polymorphism of genes encoding Kunitz-type protease inhibitors in the genus Solanum. Biochimie 94:1687–1696
Srinivasan A, Giri AP, Gupta VS (2006) Structural and functional diversities in lepidopteran serine proteases. Cell Mol Biol Lett 11:132–154
Srinivasan T, Kumar KRR, Kirti PB (2009) Constitutive expression of a trypsin protease inhibitor confers multiple stress tolerance in transgenic tobacco. Plant Cell Physiol 3:541–553
Trewavas A (2009) What is plant behavior? Plant Cell Environ 32:606–616
Walsh TA, Twitchell WP (1991) Two Kunitz-type proteinase inhibitors from potato tubers. Plant Pysiol 97:15–18
Wang KJ, Takahata Y, Kono Y, Kaizuma N (2008) Allelic differentiation of Kunitz trypsin inhibitor in wild soybean (Glycine soja). Theor Appl Genet 117:565–573
Wang L, Zhao F, Lil M, Zhang H, Gao Y, Cao P, Pan X, Wang Z, Chang W (2011) Conformational changes of rBTI from buckwheat upon binding to trypsin: implications for the role of the P89 residue in the potato inhibitor I family. PLoS One 6:1–7
Wang KJ, Li XH, Yamashita T, Takahata Y (2012) Single nucleotide mutation leading to an amino acid substitution in the variant Tik soybean Kunitz trypsin inhibitor (SKTI) identified in Chinese wild soybean (Glycine soja Sieb. & Zucc.). Plant Syst Evol 298:1–7
White WH, Erwin TL, Viator BJ (2012) Leptotrachelus dorsalis (coleoptera: carabidae): a candidate biological control agent of the sugarcane borer in Louisiana. Fla Entomol 95:261–267
World Health Organization (2009) Dengue and dengue haemorrhagic fever. Fact Sheet 117
Wu WM, Li HB, Navaneetham D, Reichenbach ZW, Tuma RF, Walsh PN (2012) The Kunitz protease inhibitor domain of protease nexin-2 inhibits factor XIa and murine carotid artery and middle cerebral artery thrombosis. Blood 120:671–677
Xu Z, Teng W, Chye M (2004) Inhibition of endogenous trypsin- and chymotrypsin-like activities in transgenic lettuce expressing heterogeneous proteinase inhibitor SaPIN2a. Planta 218:623–629
Acknowledgments
The research was supported by Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant no. 471848/2012-3) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, grant no. E-26/110.942/2013). S.B.J. was recipient of a PhD fellowship from Universidade Estadual do Norte Fluminense (UENF) and V.A.P. was recipient of a PhD fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We thank Dr. Turán Peter Ürmenyi, Universidade Federal do Rio de Janeiro (UFRJ) for critical reading of this manuscript.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Fig. S1 a SDS-PAGE (10 %) analysis of crude leaf extracts of plants exposed to MeJa by 48 and 96 h. b Gelatin-SDS-PAGE analyses (under semi-denaturing condition) of crude leaf extracts from exposed plants to MeJa by 48 and 96 h. For each lane were loaded 5 μg of protein. Trypsin inhibitory activities are shown by protein bands in a clear background (b, see Materials and methods).
Supplementary Fig. S2 a Native-PAGE (10 %) analysis of crude leaf extracts of plants exposed to MeJa by 48 and 96 h. b Gelatin-Native -PAGE analyses of crude leaf extracts from exposed plants to MeJa by 48 and 96 h. For each lane were loaded 5 μg of protein. Trypsin inhibitory activities are shown by protein bands in a clear background (b, see Materials and methods)
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Botelho-Júnior, S., Machado, O.L.T., Fernandes, K.V.S. et al. Defense response in non-genomic model species: methyl jasmonate exposure reveals the passion fruit leaves’ ability to assemble a cocktail of functionally diversified Kunitz-type trypsin inhibitors and recruit two of them against papain. Planta 240, 345–356 (2014). https://doi.org/10.1007/s00425-014-2085-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-014-2085-3