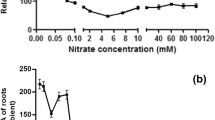
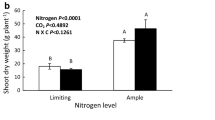
Abstract
Elevated carbon dioxide (CO2) has been shown to enhance the growth and development of plants, especially of roots. Amongst them, lateral roots play an important role in nutrient uptake, and thus alleviate the nutrient limitation to plant growth under elevated CO2. This paper examined the mechanism underlying CO2 elevation-induced lateral root formation in tomato. The endogenous nitric oxide (NO) in roots was detected by the specific probe 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM DA). We suggest that CO2 elevation-induced NO accumulation was important for lateral root formation. Elevated CO2 significantly increased the activity of nitric oxide synthase in roots, but not nitrate reductase activity. Moreover, the pharmacological evidence showed that nitric oxide synthase rather than nitrate reductase was responsible for CO2 elevation-induced NO accumulation. Elevated CO2 enhanced the activity of nitric oxide synthase and promoted production of NO, which was involved in lateral root formation in tomato under elevated CO2.





Similar content being viewed by others
Abbreviations
- cPTIO:
-
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAF-FM DA:
-
4-Amino-5-methylamino-2′,7′-difluorofluorescein diacetate
- l-NAME:
-
N G-Nitro-l-arginine methyl ester
- LR:
-
Lateral root
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- NR:
-
Nitrate reductase
- SNP:
-
Sodium nitroprusside
References
Asai S, Ohta K, Yoshioka H (2008) MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 20:1390–1406
BassiriRad H, Gutschick VP, Lussenhop J (2001) Root system adjustments: regulation of plant nutrient uptake and growth responses to elevated CO2. Oecologia 126:305–320
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Bethke PC, Gubler F, Jacobsen JV, Jones RL (2004) Dormancy of Arabidopsis seeds and barley grains can be broken by nitric oxide. Planta 219:847–855
Bethke PC, Libourel IGL, Aoyama N, Chung YY, Still DW, Jones RL (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143:1173–1188
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Bunce JA (1994) Responses of respiration to increasing atmospheric carbon-dioxide concentrations. Physiol Plant 90:427–430
Corpas FJ, Barroso JB, Carreras A, Valderrama R, Palma JM, León AM, Sandalio LM, Del Río LA (2006) Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta 224:246–254
Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218:900–905
Crawford NM (2006) Mechanisms for nitric oxide synthesis in plants. J Exp Bot 57:471–478
De Souza AP, Gaspar M, Da Silva EA, Ulian EC, Waclawovsky AJ, Nishiyama MY, Dos Santos RV, Teixeira MM, Souza GM, Buckeridge MS (2008) Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant Cell Environ 31:1116–1127
Dean JV, Harper JE (1986) Nitric oxide and nitrous oxide production by soybean and winged bean during the in vivo nitrate reductase assay. Plant Physiol 82:718–723
Ding L, Zhang J (2012) Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin 33:75–81
Du ST, Zhang YS, Lin XY, Wang Y, Tang CX (2008) Regulation of nitrate reductase by nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.). Plant Cell Environ 31:195–204
Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci USA 108:18506–18511
Ferrario-Méry S, Thibaud MC, Betsche T, Valadier MH, Foyer CH (1997) Modulation of carbon and nitrogen metabolism, and of nitrate reductase, in untransformed and transformed Nicotiana plumbaginifolia during CO2 enrichment of plants grown in pots and in hydroponic culture. Planta 202:510–521
Flores T, Todd DC, Tovar-Mendez A, Dhanoa PK, Correa-Aragunde N, Hoyos ME, Brownfield DM, Mullen RT, Lamattina L, Polacco JC (2008) Arginase-negative mutants of Arabidopsis exhibit increased nitric oxide signaling in root development. Plant Physiol 147:1936–1946
Fonseca F, Bowsher C, Stulen I (1997) Impact of elevated atmospheric carbon dioxide on nitrate reductase transcription and activity in leaves and roots of Plantago major. Physiol Plant 100:940–948
Foresi NP, Laxalt LM, Tonón CV, Casalongué CA, Lamattina L (2007) Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol 145:589–592
Geiger M, Walch-Liu P, Engels C, Harnecker J, Schulze ED, Ludewig F, Sonnewald U, Scheible WR, Stitt M (1998) Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant Cell Environ 21:253–268
Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17:3436–3450
Himanen K, Boucheron E, Vanneste S, Engler JD, Inzé D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14:2339–2351
Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ (2009a) Elevated carbon dioxide improves plant iron nutrition through enhancing the iron-deficiency-induced responses under iron-limited conditions in tomato. Plant Physiol 150:272–280
Jin CW, Du ST, Zhang YS, Lin XY, Tang CX (2009b) Differential regulatory role of nitric oxide in mediating nitrate reductase activity in roots of tomato (Solanum lycocarpum). Ann Bot 104:9–17
Jin CW, Du ST, Shamsi IH, Luo BF, Lin XY (2011) NO synthase-generated NO acts downstream of auxin in regulating Fe-deficiency-induced root branching that enhances Fe-deficiency tolerance in tomato plants. J Exp Bot 62:3875–3884
Kolbert Z, Erdei L (2008) Involvement of nitrate reductase in auxin-induced NO synthesis. Plant Signal Behav 3:972–973
Lamattina L, García-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54:109–136
Lee-Ho E, Walton LJ, Reid DM, Yeung EC, Kurepin LV (2007) Effects of elevated carbon dioxide and sucrose concentrations on Arabidopsis thaliana root architecture and anatomy. Can J Bot 85:324–330
Li CR, Gan LJ, Xia K, Zhou X, Hew CS (2002) Responses of carboxylating enzymes, sucrose metabolizing enzymes and plant hormones in a tropical epiphytic CAM orchid to CO2 enrichment. Plant Cell Environ 25:369–377
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44
Matt P, Geiger M, Walch-Liu P, Engels C, Krapp A, Stitt M (2001) Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant Cell Environ 24:1119–1137
Mayer B, Hemmens B (1997) Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem Sci 22:477–481
Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye A, Gregory J et al (2007) Global climate projections. In: Solomon S, Qin D, Manning M et al (eds) Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, UK, pp 747–845
Méndez-Bravo A, Raya-González J, Herrera-Estrella L, López-Bucio J (2010) Nitric oxide is involved in alkamide-induced lateral root development in Arabidopsis. Plant Cell Physiol 51:1612–1626
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:11–35
Neill SJ, Hancock JT, Desikan R (2006) Preface to nitric oxide signalling: plant growth and development. J Exp Bot 57:462
Niu YF, Jin CW, Jin GL, Zhou QY, Lin XY, Tang CX, Zhang YS (2011) Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2. Plant Cell Environ 34:1304–1317
Pasternak T, Rudas V, Potters G, Jansen MAK (2005) Morphogenic effects of abiotic stress: reorientation of growth in Arabidopsis thaliana seedlings. Environ Exp Bot 53:299–314
Planchet E, Sonoda M, Zeier J, Kaiser WM (2006) Nitric oxide (NO) as an intermediate in the cryptogein-induced hypersensitive response—a critical re-evaluation. Plant Cell Environ 29:59–69
Prior SA, Torbert HA, Runion GB, Mullins GL, Rogers HH, Mauney JR (1998) Effects of carbon dioxide enrichment on cotton nutrient dynamics. J Plant Nutri 21:1407–1426
Teng NJ, Wang J, Chen T, Wu XQ, Wang YH, Lin JX (2006) Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol 172:92–103
Tian QY, Sun DH, Zhao MG, Zhang WH (2007) Inhibition of nitric oxide synthase (NOS) underlies aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol 174:322–331
Wang Y, Du ST, Li LL, Huang LD, Fang P, Lin XY, Zhang YS, Wang HL (2009) Effect of CO2 elevation on root growth and its relationship with indole acetic acid and ethylene in tomato seedlings. Pedosphere 19:570–576
Xiong J, Lu H, Lu KX, Duan YX, An LY, Zhu C (2009) Cadmium decreases crown root number by decreasing endogenous nitric oxide, which is indispensable for crown root primordia initiation in rice seedlings. Planta 230:599–610
Acknowledgments
This work was financially supported by the Project of Transformation Fund for Agricultural Scientific and Technological Achievements of China [2010GB23600669] and the State Key Development Program for Basic Research of China [973 Program, no. 2009CB119003].We are thankful to the 985-Institute of Agrobiology and Environmental Sciences of Zhejiang University for providing the experimental equipment. CT was supported by Australian Research Council through the Linkage Projects funding scheme [LP100200757].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Xiao, W., Niu, Y. et al. Nitric oxide enhances development of lateral roots in tomato (Solanum lycopersicum L.) under elevated carbon dioxide. Planta 237, 137–144 (2013). https://doi.org/10.1007/s00425-012-1763-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-012-1763-2