Abstract
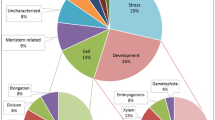
Potato (Solanum tuberosum) tubers are underground storage organs covered by the skin or periderm, a suberized layer that protects inner flesh from dehydration and pathogens. Understanding the molecular processes associated with periderm formation is of great importance for a better knowledge of this protective tissue and for improving the storage life of tubers. Here, to isolate new candidate genes for potato periderm, a suppression subtractive hybridization library from potato skin was performed. This library yielded a comprehensive list of 108 candidate genes that were manually sorted in functional categories according to the main cellular and metabolic processes in periderm. As expected, the list contains Suberin and wax genes, including some genes with a demonstrated role in the biosynthesis of these cell wall aliphatic compounds. Moreover, Regulation and Stress and defence genes are highly abundant in the library in general agreement with previous potato skin proteomic studies. The putative function of the genes in periderm is discussed.




Similar content being viewed by others
Abbreviations
- ESTs:
-
Expressed sequence tag
- SSH:
-
Suppression subtractive hybridization
- POCI:
-
Potato Oligo Chip Initiative
- StKCS6:
-
Solanum tuberosum 3-Ketoacyl-CoA-synthase
- FHT:
-
Fatty ω-hydroxyacid/fatty alcohol hydroxycinnamoyl transferase
- LACS:
-
Long-chain acyl-CoA synthethase
- PAL:
-
Phenylalanine ammonia lyase
- F5H:
-
Ferulate 5 hydroxylase
- ROS:
-
Reactive oxygen species
- DEM:
-
Defective embryo meristem
- PCD:
-
Programmed cell death
- LTP:
-
Lipid transfer protein
- IQR:
-
Interquartile range
- Ct:
-
Threshold cycle
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- PCA:
-
Principal component analysis
- NCBI:
-
National Center for Biotechnology Information
- TAIR:
-
The Arabidopsis Information Resource
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bajji M, M’Hamdi M, Gastiny F, Delaplace P, Fauconnier ML, du Jardin P (2007) Catalase inhibition alters suberization and wound-healing in potato (Solanum tuberosum L.) tubers. Physiol Plant 129:472–483
Bajla I, Holländer I, Fluch S, Burg K, Kollár M (2005) An alternative method for electrophoretic gel image analysis in the GelMaster software. Comput Methods Programs Biomed 77:209–231
Barel G, Ginzberg I (2008) Potato skin proteome is enriched with plant defence components. J Exp Bot 59:3347–3357
Bernards MA (2002) Demystifying suberin. Can J Bot 80:227–240
Bernards MA, Razem FA (2001) The poly(phenolic) domain of potato suberin: a non-lignin cell wall bio-polymer. Phytochem 57:1115–1122
Bernards MA, Fleming WD, Llewellyn DB, Priefer R, Yang X, Sabatino A, Plourde GL (1999) Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiol 121:135–146
Bernards MA, Susag LM, Bedgar DL, Anterola AM, Lewis NG (2000) Induced phenylpropanoid metabolism during suberization and lignification: a comparative analysis. J Plant Physiol 157:601–607
Bernier F, Berna A (2001) Germins and germin-like proteins: plant do-all proteins. But what do they do exactly? Plant Physiol Biochem 39:545–554
Buchanan BB, Balmer Y (2005) Redox regulation: a broadening horizon. Annu Rev Plant Biol 56:187–220
Caliskan M, Cuming AC (1998) Spatial specificity of H2O2-generating oxalate oxidase gene expression during wheat embryo germination. Plant J 15:165–171
Cannon MC, Terneus K, Hall Q, Tan L, Wang Y, Wegenhart BL, Chen L, Lamport DT, Chen Y, Kieliszewski MJ (2008) Self-assembly of the plant cell wall requires an extensin scaffold. Proc Natl Acad Sci USA 105:2226–2231
Chaves I, Pinheiro C, Paiva JA, Planchon S, Sergeant K, Renaut J, Graça JA, Costa G, Coelho AV, Ricardo CP (2009) Proteomic evaluation of wound-healing processes in potato (Solanum tuberosum L.) tuber tissue. Proteomics 9:4154–4175
Davletova S, Rizhsky L, Liang H, Zhong S, Oliver D, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17:268–281
De Smet I, Zhang H, Inzé D, Beeckman T (2006) A novel role for abscisic acid emerges from underground. Trends Plant Sci 11:434–439
DeBono A, Trevor H, Yeats TH, Rose JKC, Bird D, Jetter R, Kunst L, Samuels L (2009) Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. Plant Cell 21:1230–1238
Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED, Siebert PD (1996) Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci USA 93:6025–6030
Dunwell JM, Gibbings JG, Mahmood T, Naqvi SMS (2008) Germin and germin-like proteins: evolution, structure, and function. Crit Rev Plant Sci 27:342–375
Espelie KE, Kolattukudy PE (1985) Purification and characterization of an abscisic acid-inducible anionic peroxidase associated with suberization in potato (Solanum tuberosum). Arch Biochem Biophys 240:539–545
Espelie KE, Franceschi VR, Kolattukudy PE (1986) Immunocytochemical localization and time course of appearance of an anionic peroxidase associated with suberization in wound-healing potato tuber tissue. Plant Physiol 81:487–492
Ewing B, Green P (1998) Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res 8:186–194
Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8:175–185
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Franke R, McMichael CM, Meyer K, Shirley AM, Cusumano JC, Chapple C (2000) Modified lignin in tobacco and poplar plants over-expressing the Arabidopsis gene encoding ferulate 5-hydroxylase. Plant J 22:223–234
Franke R, Höfer R, Briesen I, Emsermann M, Efremova N, Yephremov A, Schreiber L (2009) The DAISY gene from Arabidopsis encodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds. Plant J 57:80–95
Fry SC (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol 161:641–675
Ginzberg I, Barel G, Ophir R, Tzin E, Tanami Z, Muddarangappa T, de Jong W, Fogelman E (2009) Transcriptomic profiling of heat-stress response in potato periderm. J Exp Bot 60:4411–4421
Graça J, Santos S (2007) Suberin: a biopolyester of plants’ skin. Macromol Biosci 7:128–135
Hrmova M, Fincher GB (2001) Structure-function relationships of β-d-glucan endo- and exohydrolases from higher plants. Plant Mol Biol 47:73–91
Huang X, Li Y, Niu Q, Zhang K (2007) Suppression Subtractive Hybridization (SSH) and its modifications in microbiological research. Appl Microbiol Biotechnol 76:753–760
Israelsson M, Siegel RS, Young J, Hashimoto M, Iba K, Schroeder JI (2006) Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr Opin Plant Biol 9:654–663
Iwai H, Masaoka N, Ishii T, Satoh S (2002) A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proc Natl Acad Sci USA 99:16319–16324
Kariola T, Brader G, Helenius E, Li J, Heino P, Palva ET (2006) EARLY RESPONSIVE TO DEHYDRATION 15, a negative regulator of abscisic acid responses in Arabidopsis. Plant Physiol 142:1559–1573
Keddie JS, Carroll BJ, Thomas CM, Reyes ME, Klimyuk V, Holtan H, Gruissem W, Jones JD (1998) Transposon tagging of the Defective embryo and meristems gene of tomato. Plant Cell 10:877–888
Kloosterman B, De Koeyer D, Griffiths R, Flinn B, Steuernagel B, Scholz U, Sonnewald S, Sonnewald U, Bryan GJ, Prat S, Bánfalvi Z, Hammond JP, Geigenberger P, Nielsen KL, Visser RG, Bachem CW (2008) Genes driving potato tuber initiation and growth: identification based on transcriptional changes using the POCI array. Funct Integr Genomics 8:329–340
Kolattukudy P (1981) Structure, biosynthesis, and biodegradation of cutin and suberin. Annu Rev Plant Physiol 32:539–567
Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK (2009) The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planta 230:119–134
Kumar GNM, Lulai EC, Suttle JC, Knowles NR (2010) Age-induced loss of wound-healing ability in potato tubers is partly regulated by ABA. Planta 232:1433–1445
LeBouquin R, Skrabs M, Kahn R, Benveniste I, Salaün JP, Schreiber L, Durst F, Pinot F (2001) CYP94A5, a new cytochrome P450 from Nicotiana tabacum is able to catalyze the oxidation of fatty acids to the ω-alcohol and to the corresponding diacid. Eur J Biochem 268:3083–3090
Lee KP, Piskurewicza U, Turecková V, Sornad M, Lopez-Molina L (2010) A seed coat bedding assay shows that RGL2-dependent release of abscisic acid by the endosperm controls embryo growth in Arabidopsis dormant seeds. Proc Natl Acad Sci USA 107:19108–19113
Lendzian KJ (2006) Survival strategies of plants during secondary growth: barrier properties of phellems and lenticels towards water, oxygen, and carbon dioxide. J Exp Bot 57:2535–2546
Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49:199–222
Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163:16–20
Lulai EC, Suttle JC, Pederson SM (2008) Regulatory involvement of abscisic acid in potato tuber wound-healing. J Exp Bot 59:1175–1186
Morishita T, Kojima Y, Maruta T, Nishizawa-Yokoi A, Yabuta Y, Shigeoka S (2009) Arabidopsis NAC transcription factor, ANAC078, regulates flavonoid biosynthesis under high-light. Plant Cell Physiol 50:2210–2222
Nicot N, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Olsen AN, Ernst HA, Leggio LL, Skriver K (2005) NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci 10:79–87
Peterson RL, Barker WG (1979) Early tuber development from explanted stolon nodes of Solanum tuberosum var. Kennebec. Bot Gaz 140:398–406
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Pla M, Huguet G, Verdaguer D, Puigderrajols P, Llompart B, Nadal A, Molinas M (1998) Stress proteins co-expressed in suberized and lignified cells and in apical meristems. Plant Sci 139:49–57
Pla M, Jofré A, Martell M, Molinas M, Gómez J (2000) Large accumulation of mRNA and DNA point modifications in a plant senescent tissue. FEBS Lett 472:14–16
Pollard M, Beisson F, Li Y, Ohlrogge JB (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13:236–246
Reina JJ, Guerrero C, Heredia A (2007) Isolation, characterization, and localization of AgaSGNH cDNA: a new SGNH-motif plant hydrolase specific to Agave americana L. leaf epidermis. J Exp Bot 58:2717–2731
Roberts E, Kolattukudy PE (1989) Molecular cloning, nucleotide sequence, and abscisic-acid induction of a suberization-associated highly anionic peroxidase. Mol Gen Genet 217:223–232
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Sabba RP, Lulai EC (2002) Histological analysis of the maturation of native and wound periderm in potato (Solanum tuberosum L.) tuber. Ann Bot 90:1–10
Schreiber L (2010) Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci 15:546–553
Schreiber L, Franke R, Hartmann K (2005) Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta 220:520–530
Serra O, Soler M, Hohn C, Franke R, Schreiber L, Prat S, Molinas M, Figueras M (2009a) Silencing of StKCS6 in potato periderm leads to reduced chain lengths of suberin and wax compounds and increased peridermal transpiration. J Exp Bot 60:697–707
Serra O, Soler M, Hohn C, Sauveplane V, Pinot F, Franke R, Schreiber L, Prat S, Molinas M, Figueras M (2009b) CYP86A33-targeted gene silencing in potato tuber alters suberin composition, distorts suberin lamellae, and impairs the periderm’s water barrier function. Plant Physiol 149:1050–1060
Serra O, Hohn C, Franke R, Prat S, Molinas M, Figueras M (2010) A feruloyl transferase involved in the biosynthesis of suberin and suberin-associated wax is required for maturation and sealing properties of potato periderm. Plant J 62:277–290
Sherf BA, Bajar AM, Kolattukudy PE (1993) Abolition of an inducible highly anionic peroxidase-activity in transgenic tomato. Plant Physiol 101:201–208
Soler M, Serra O, Molinas M, Huguet G, Fluch S, Figueras M (2007) A genomic approach to suberin biosynthesis and cork differentiation. Plant Physiol 144:419–431
Soler M, Serra O, Molinas M, García-Berthou E, Caritat A, Figueras M (2008) Seasonal variation in transcript abundance in cork tissue analyzed by real time RT-PCR. Tree Physiol 28:743–751
Tian L, Chen ZJ (2001) Blocking histone deacetylation in Arabidopsis induces pleiotropic effects on plant gene regulation and development. Proc Natl Acad Sci USA 98:200–205
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, Van Montagu M, Inzé D, Van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16:4806–4816
Wong HL, Sakamoto T, Kawasaki T, Umemura K, Shimamoto K (2004) Down-regulation of metallothionein, a reactive oxygen scavenger, by the small GTPase OsRac1 in rice. Plant Physiol 135:1447–1456
Xiong L, Ishitani M, Lee H, Zhu JK (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13:206–2083
Yamaguchi-Shinozaki K, Shinozaki K (1993) The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of rd22, a gene responsive to dehydration stress in Arabidopsis thaliana. Mol Gen Genet 238:17–25
Yang WL, Bernards MA (2007) Metabolite profiling of potato (Solanum tuberosum L.) tubers during wound-induced suberization. Metabolomics 3:147–159
Acknowledgments
The authors would like to thank the Laboratory of Genetics Ichthyology (Biology Department, University of Girona) for kindly lending the Real time Thermocycler. The authors are very grateful to Mr. J. Falgàs for providing potato material. This work was supported by funding from Spanish Ministerio de Ciencia y Tecnología (AGL2003-00416) and from the Ministerio de Innovación y Ciencia (AGL2009-13745).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2011_1350_MOESM3_ESM.tif
Supplemental Fig. S1 Variation in the expression of six housekeeping genes in potato periderm and parenchymatic flesh. Threshold cycle (Ct) variation obtained by real time RT-PCR is presented as a box-and-whisker plot. Boxes indicate the 25/75 percentiles, and the line inside each square indicates the median. Whisker caps indicate 1.5-fold the interquartile range (IQR), and outliers are indicated by dots. Cyclophilin shows both the lowest standard deviation and lowest Ct values (TIFF 96 kb)
Rights and permissions
About this article
Cite this article
Soler, M., Serra, O., Fluch, S. et al. A potato skin SSH library yields new candidate genes for suberin biosynthesis and periderm formation. Planta 233, 933–945 (2011). https://doi.org/10.1007/s00425-011-1350-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1350-y