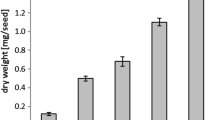
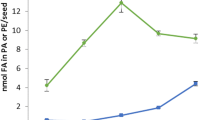
Abstract
The major fatty acid component of castor (Ricinus communis L.) oil is ricinoleic acid (12-hydroxy-cis-9-octadecenoic acid), and unsaturated hydroxy acid accounts for >85% of the total fatty acids in triacylglycerol (TAG). TAG had a higher ricinoleate content at position 2 than at positions 1 and 3. Although lysophosphatidic acid (LPA) acyltransferase (EC 2.3.1.51), which catalyzes acylation of LPA at position 2, was expected to utilize ricinoleoyl-CoA preferentially over other fatty acyl-CoAs, no activity was found for ricinoleoyl-CoA in vitro at concentrations at which other unsaturated acyl-CoAs were incorporated rapidly. However, activity for ricinoleoyl-CoA appeared with addition of polyamines (putrescine, spermidine, and spermine), while polyamines decreased the rates of incorporation of other acyl-CoAs into position 2. The order of effect of polyamines on LPA acyltransferase activity was spermine > spermidine >> putrescine. At concentrations of spermine and spermidine of >0.1 mM, ricinoleoyl-CoA served as an effective substrate for LPA acyltransferase reaction. The concentrations of spermine and spermidine in the developing seeds were estimated at ∼0.09 and ∼0.63 mM, respectively. These stimulatory effects for incorporation of ricinoleate were specific to polyamines, but basic amino acids were ineffective as cations. In contrast, in microsomes from safflower seeds that do not contain ricinoleic acid, spermine and spermidine stimulated the LPA acyltransferase reaction for all acyl-CoAs tested, including ricinoleoyl-CoA. Although the fatty acid composition of TAG depends on both acyl-CoA composition in the cell and substrate specificity of acyltransferases, castor bean polyamines are crucial for incorporation of ricinoleate into position 2 of LPA. Polyamines are essential for synthesis of 2-ricinoleoyl phosphatidic acid in developing castor seeds.








Similar content being viewed by others
Abbreviations
- G3P:
-
Glycerol 3-phosphate
- LPA:
-
Lysophosphatidic acid
- PA:
-
Phosphatidic acid
- DAG:
-
Diacylglycerol
- TAG:
-
Triacylglycerol
- DAF:
-
Days after flowering
References
Achaya KT, Craig BM, Youngs CG (1964) The component fatty acids and glycerides of castor oil. J Am Oil Chem Soc 41:783–784
Atsmon D (1989) Castor. In: Robbelen G, Downey KR (eds) Oil crops of the world. McGraw-Hill, New York, pp 438–447
Bafor M, Smith MA, Jonsson L, Stobart K, Stymne S (1991) Ricinoleic acid biosynthesis and triacylglycerol assembly in microsomal preparations from developing castor bean (Ricinus communis) endosperm. Biochem J 280:507–514
Bagni N, Pistocchi R (1992) Polyamine metabolism and compartmentation in plant cell. In: Mengal K, Pilbeam DJ (eds) Nitrogen metabolism of plants. Clarendon Press, Oxford, pp 229–248
Bertrams M, Heinz E (1981) Positional specificity and fatty acid selectivity of purified sn-glycerol 3-phosphate acyltransferases from chloroplasts. Plant Physiol 68:653–657
Brockerhoff H, Yurkowski M (1966) Stereospecific analyses of several vegetable fats. J Lipid Res 7:62–64
Broun P, Somerville C (1997) Accumulation of ricinoleic, lesquerolic, and densipolic acids in seeds of transgenic Arabidopsis plants that express a fatty acyl hydroxylase cDNA from castor bean. Plant Physiol 113:933–942
Cao Y, Huang AHC (1987) Acyl Coenzyme: a preference of diacylglycerol acyltransferase from the maturing seeds of Cuphea, maize, rapeseed, and canola. Plant Physiol 84:762–765
Carman GM, Harrington GF, Amegah R (1981) Microsomal-associated glycerophosphate acyltransferase activity in germinating soybeans. J Food Biochem 5:185–196
Dahlqvist A, Stål U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne S (2000) Phospholipid:diacylglycerolacyltransferase: an enzyme that catalyzes the acyl-CoA-Independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97:6487–6492
Dittmer JC, Lester RL (1964) A simple, specific spray for the detection of phospholipids on thin-layer chromatograms. J Lipid Res 5:126–127
Erhan SZ, Adhvaryu A (2005) Non-food lipids. In Murphy DJ (ed) Plant lipids. Blackwell, Oxford, pp 103–122
Evans PT, Malmberg RL (1989) Do polyamines have roles in plant development? Annu Rev Plant Physiol Mol Biol 40:235–269
Flores HE, Young ND, Galston AW (1985) Polyamine metabolism and plant stress. In: Key JL, Kosuge T (eds) Cellular and molecular biology of plant stress. Alan R. Liss, New York, pp 93–114
Flores HE, Protacio CM, Signs MW (1989) Primary and secondary metabolism of polyamines in plants. In: Poulton JE, Romeo JT, Conn EE (eds) Plant Nitrogen Metabolism. Rec. Adv. Phytochem, vol 23. Plenum Press, New York, pp 329–393
Fores EH, Galston AW (1982) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol 69:701–706
Frentzen M, Heinz E, Mckeon TA, Stumpf PK (1983) Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem 129:629–636
Galston AW, Sawhney RK (1990) Polyamines in plant physiology. Plant Physiol 94:406–410
Gamarnik A, Frydman RB (1991) Cadaverine, an essential diamine for the normal root development of germinating soybean (Glycine max) seeds. Plant Physiol 97:778–785
Griffiths G, Stobart AK, Stymne S (1985) The acylation of sn-glycerol 3-phosphate and the metabolism of phosphatidate in microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius L.) seeds. Biochem J 230:379–388
Hatje G (1989) World importance of oil crops and their products. In: Robbelen G, Downey KR (eds) Oil crops of the world. McGraw-Hill, New York, pp 1–21
Ichihara K (1984) sn-Glycerol-3-phosphate acyltransferase in a particulate fraction from maturing safflower seeds. Arch Biochem Byophys 232:685–698
Ichihara K, Noda M (1980) Fatty acid composition and lipid synthesis in developing safflower seeds. Phytochemistry 19:49–54
Ichihara K, Noda M (1982) Some properties of diacylglycerol acyltransferase in a particulate fraction from maturing safflower seeds. Pytochemistry 21:1895–1901
Ichihara K, Asahi T, Fujii S (1987) 1-Acyl-sn-glycerol-3-phosphate acyltransferase in maturing safflower seeds and its contribution to the non-random fatty acid distribution of triacylglycerol. Eur J Biochem 167:339–347
Jamdar SC (1979) Hepatic lipid metabolism: effect of spermine, albumin, and Z protein on microsomal lipid formation. Arch Biochem Biophys 195:81–94
Kennedy EP (1961) Biosynthesis of complex lipids. Fed Proc Am Soc Exp Biol 3:2250–2254
Kim HU, Huang AHC (2004) Plastid lysophosphatidic acid acyltransferase is essential for enbryo development in Arabidopsis. Plant Physiol 134:1206–1216
Lin JT, Lew KM, Chen JM, Iwasaki Y, Mckeon TA (2000) Metabolism of 1-acyl-2-oleoyl-sn-glycerol-3-phosphoethanolamine in castor oil biosynthesis. Lipids 35:481–486
Liquori AM, Costantino L, Crescenzi V, Elia V, Giglio E, Puliti R, De Santis Savino M, Vitagliano V (1967) Complexes between DNA and polyamines: a molecular model. J Mol Biol 24:113–122
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Maisonneuve S, Guyot R, Delseny M, Roscoe T (2003) A multigene family of lysophosphatidic acid acyltransferases of Arabidopsis thaliana. In: Murata K, Yamada M, Nishida I, Okuyama H, Sekiya J, Hajime W (eds) Advanced research on plant lipids. Kluwer, Dordrecht, pp 183–186
Mchenry L, Fritz PJ (1987) Cocoa butter biosynthesis: effect of temperature on Theobroma cacao acyltransferases. J Am Oil Chem Soc 64:1012–1015
Mckeon TA, Chen GQ, Lin JT (2000) Biochemical aspects of castor oil biosynthesis. Biochem Soc Trans 28:972–974
Pryde EH, Rothfus JA (1989) Industrial and nonfood uses of vegetable oils. In: Robbelen G, Downey KR (eds) Oil crops of the world. McGraw-Hill, New York, pp 87–117
Redmond JW, Tseng A (1979) High-pressure liquid chromatographic determination of putrescine, cadaverine, spermidine and spermine. J Chromatogr 170:479–481
Shine WE, Mancha M, Stumpf PK (1976) Fat metabolism in higher plants. Differential incorporation of acyl-Coenzymes A and acyl-acyl carrier proteins into plant microsomal lipids. Arch Biochem Biophys 173:472–479
Smith TA (1975) Recent advances in the biochemistry of plant amines. Phytochemistry 14:865–890
Smith TA, Bagni N, Fracassini DS (1979) The formation of amines and their derivatives in plants. In: Hewitt EJ, Cutting CV (eds) Nitrogen assimilation of plants. Academic, New York, pp 557–570
Smith MA, Chowrira G, Kunst L (2003) Production of hydroxy fatty acids in Arabidopsis. In: Murata N, Yamada M, Nishida I, Okuyama H, Sekiya J, Hajime W (eds) Advanced research on plant lipids. Kluwer, Dordrecht, pp 427–430
Stål U, Banas A, Stymne S (1995) Plant microsomal phospholipids hydrolases have selectivities for uncommon fatty acids. Plant Physiol 107:953–962
Urano K, Hobo T, Shinozaki K (2005) Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed development. FEBS Lett 579:1557–1564
Vallari DS, Rock LO (1982) Role of spermidine in the activity of sn-glycerol-3-phosphate acyltransferase from Escherichia Coli. Arch Biochem Biophys 218:402–408
Van de Loo FJ, Broun P, Turner S, Somerville C (1995) An oleate 12-hydroxylase from Ricinus communis L. is a fatty acyl desaturase homolog. Proc Natl Acad Sci USA 92:6743–6747
Vick B, Beevers H (1977) Phosphatidic acid synthesis in castor bean endosperm. Plant Physiol 59:459–463
Vogel G, Browse J (1996) Cholinephosphotransferase and diacylglycerol acyltransferase. Plant Physiol 110:923–931
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomosugi, M., Ichihara, K. & Saito, K. Polyamines are essential for the synthesis of 2-ricinoleoyl phosphatidic acid in developing seeds of castor. Planta 223, 349–358 (2006). https://doi.org/10.1007/s00425-005-0083-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0083-1