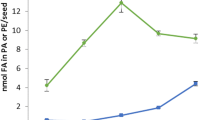
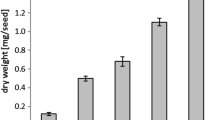
Abstract
We have examined the role of 2-oleoyl-PE (phosphatidylethanolamine) in the biosynthesis of triacylglycerols (TAG) by castor microsomes. In castor microsomal incubation, the label from 14C-oleate of 1-palmitoyl-2-[1-14C]oleoyl-sn-glycero-3-phosphoethanolamine is incorporated into TAG containing ricinoleate. The enzyme characteristics, such as optimal pH, and the effect of incubation components of the oleoyl-12-hydroxylase using 2-oleoyl-PE as incubation substrate are similar to those for 2-oleoyl-PC (phosphatidylcholine). However, compared to 2-oleoyl-PC, 2-oleoyl-PE is a less efficient incubation substrate of oleoyl-12-hydroxylase in castor microsomes. Unlike 2-oleoyl-PC, 2-oleoyl-PE is not hydroxylated to 2-ricinoleoyl-PE by oleoyl-12-hydroxylase and is not desaturated to 2-linoleoyl-PE by oleoyl-12-desaturase. We have demonstrated the conversion of 2-oleoyl-PE to 2-oleoyl-PC and vice versa. The incorporation of label from 2-[14C]oleoyl-PE into TAG occurs after its conversion to 2-oleoyl-PC, which can then be hydroxylated or desaturated. We detected neither PE-N-monomethyl nor PE-N,N-dimethyl, the intermediates from PE to PC by N-methylation. The conversion of 2-oleoyl-PE to 2-oleoyl-PC likely occurs via hydrolysis to 1,2-diacyl-sn-glycerol by phospholipase C and then by cholinephosphotransferase. This conversion does not appear to play a key role in driving ricinoleate into TAG.
Similar content being viewed by others
Abbreviations
- DAG:
-
diacylglycerol
- FA:
-
fatty acid
- FAME:
-
fatty acid methyl ester
- FFA:
-
free fatty acid
- HPLC:
-
high-performance liquid chromatography
- 2-linoleoyl-PC:
-
1-acyl-2-linoleoyl-sn-glycero-3-phosphocholine
- 2-oleoyl-PC:
-
1-acyl-2-oleoyl-sn-glycero-3-phosphocholine
- 2-oleoyl-PE:
-
1-acyl-2-oleoyl-sn-glycero-3-phosphoethanolamine
- PC:
-
phosphatidylcholine
- PE:
-
phosphatidylethanolamine
- 2-ricinoleoyl-PC:
-
1-acyl-2-ricinoleoyl-sn-glycero-3-phosphocholine
- 2-ricinoleoyl-PE:
-
1-acyl-2-ricinoleoyl-sn-glycero-3-phosphoethanolamine
- TAG:
-
triacylglycerol
References
van de Loo, F.J., Broun, P., Turner, S., and Somerville, C. (1995) An Oleate 12-Hydroxylase from Ricinus communis L. Is a Fatty Acyl Desaturase Homolog, Proc. Natl. Acad. Sci. USA 92, 6743–6747.
Broun, P., and Somerville, C. (1997) Accumulation of Ricinoleic, Lesquerolic, and Densipolic Acids in Seeds of Transgenic Arabidopsis Plants That Express a Fatty Acyl Hydroxylase cDNA from Castor Bean, Plant Physiol. 113, 933–942.
Lin, J.T., Woodruff, C.L., Lagouche, O.J., McKeon, T.A., Stafford, A.E., Goodrich-Tanrikulu, M., Singleton, J.A., and Haney, C.A. (1998) Biosynthesis of Triacylglycerols Containing Ricinoleate in Castor Microsomes Using 1-Acyl-2-oleoyl-sn-glycero-3-phosphocholine as the Substrate of Oleoyl-12-hydroxylase, Lipids 33, 59–69.
Paltauf, F., and Hermetter, A. (1991) Preparation of Alkyl Ether and Vinyl Ether Substrates for Phospholipases, Methods Enzymol. 197, 134–149.
Mishima, N., Mizumoto, K., Iwasaki, Y., Nakano, H., and Yamane, T. (1997) Insertion of Stabilizing Loci in Vectors of T7 RNA Polymerase-Mediated Escherichia coli Expression Systems: A Case Study on the Plasmids Involving Foreign Phospholipase D Gene, Biotechnol. Prog. 13, 864–868.
Lin, J.T., McKeon, T.A., Tanrikulu, M.G., and Stafford, A.E. (1996) Characterization of Oleoyl-12-hydroxylase in Castor Microsomes Using the Putative Substrate, 1-Acyl-2-oleoyl-sn-glycero-phosphocholine, Lipids 31, 571–577.
Singleton, J.A., and Stikeleather, L.F. (1995) High-Performance Liquid Chromatography Analysis of Peanut Phospholipids. II. Effect of Postharvest Stress on Phospholipid Composition, J. Am. Oil Chem. Soc. 72, 485–488.
Lin, J.T., McKeon, T.A., Woodruff, C.L., and Singleton, J.A. (1998) Separation of Synthetic Phosphatidylcholine Molecular Species by High-Performance Liquid Chromatography on a C8 Column, J. Chromatogr. A 824, 169–174.
Lin, J.T., Woodruff, C.L., and McKeon, T.A. (1997) Non-Aqueous Reversed-Phase High-Performance Liquid Chromatography of Synthetic Triacylglycerols and Diacylglycerols, J. Chromatogr. A 782, 41–48.
Lin, J.T., Snyder, L.R., and McKeon, T.A. (1998) Prediction of Relative Retention Times of Triacylglycerols in Non-Aqueous Reversed-Phase High-Performance Liquid Chromatography, J. Chromatogr. A 808, 43–49.
Lin, J.T., McKeon, T.A., and Stafford, A.E. (1995) Gradient Reversed-Phase High-Performance Liquid Chromatography of Saturated, Unsaturated and Oxygenated Free Fatty Acids and Their Methyl Esters, J. Chromatogr. A 699, 85–91.
Galliard, T., and Stumpf, P.K. (1966) Fat Metabolism in Higher Plants XXX. Enzymatic Synthesis of Ricinoleic Acid by a Microsomal Preparation from Developing Ricinus communis Seeds, J. Biol. Chem. 241, 5806–5812.
Bafor, M., Smith, M.A., Jonsson, L., Stobart, K., and Stymne, S. (1991) Ricinoleic Acid Biosynthesis and Triacylglycerol Assembly in Microsomal Preparations from Developing Castor Bean (Ricinus communis) Endosperm, Biochem. J. 280, 507–514.
Moreau, R.A., and Stumpf, P.K. (1981) Recent Studies of the Enzyme Synthesis of Ricinoleic Acid by Developing Castor Beans, Plant Physiol. 67, 672–676.
Moore, T.S. (1976) Phosphatidylcholine Synthesis in Castor Bean Endosperm, Plant Physiol. 57, 383–386.
Smith, M.A., Jonsson, L., Stymne, S., and Stobart, K. (1992) Evidence for Cytochrome b5 as an Electron Donor in Ricinoleic Acid Biosynthesis in Microsomal Preparations from Developing Castor Bean (Ricinus communis L.), Biochem. J. 287, 141–144.
Vogel, G., and Browse, J. (1996) Cholinephosphotransferase and Diacylglycerol Acyltransferase, Substrate Specificities at a Key Branch Point in Seed Lipid Metabolism, Plant Physiol. 110, 923–931.
Shin, S., and Moore, T.S. (1990) Phosphatidylethanolamine Synthesis by Castor Bean Endosperm, a Base Exchange Reaction, Plant Physiol. 93, 148–153.
Sparace, S.A., Wagner, L.K., and Moore, T.S. (1981) Phosphatidylethanolamine Synthesis in Castor Bean Endosperm, Plant Physiol. 67, 922–925.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lin, JT., Lew, K.M., Chen, J.M. et al. Metabolism of 1-acyl-2-oleoyl-sn-glycero-3-phosphoethanolamine in castor oil biosynthesis. Lipids 35, 481–486 (2000). https://doi.org/10.1007/s11745-000-547-5
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-000-547-5