Abstract
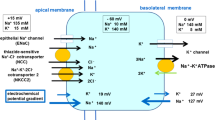
The endolymphatic sac is a small sac-shaped organ at the end of the membranous labyrinth of the inner ear. The endolymphatic sac absorbs the endolymph, in which the ion balance is crucial for inner ear homeostasis. Of the three sections of the endolymphatic sac, the intermediate portion is the center of endolymph absorption, particularly sodium transport, and is thought to be regulated by aldosterone. Disorders of the endolymphatic sac may cause an excess of endolymph (endolymphatic hydrops), a histological observation in Meniere’s disease. A low-salt diet is an effective treatment for Meniere’s disease, and is based on the assumption that the absorption of endolymph in the endolymphatic sac abates endolymphatic hydrops through a physiological increase in aldosterone level. However, the molecular basis of endolymph absorption in each portion of the endolymphatic sac is largely unknown because of difficulties in gene expression analysis, resulting from its small size and intricate structure. The present study combined reverse transcription-quantitative polymerase chain reaction and laser capture microdissection techniques to analyze the difference of gene expression of the aldosterone-controlled epithelial Na+ channel, thiazide-sensitive Na+-Cl− cotransporter, and Na+, K+-ATPase genes in the three individual portions of the endolymphatic sac in a rat model. A low-salt diet increased the expression of aldosterone-controlled ion transporters, particularly in the intermediate portion of the endolymphatic sac. Our findings will contribute to the understanding of the physiological function of the endolymphatic sac and the pathophysiology of Meniere’s disease.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Code availability
Not applicable.
References
Akiyama K, Miyashita T, Matsubara A, Inamoto R, Mori T, Nishiyama A, Mori N (2010) Expression and localization of 11beta-hydroxysteroid dehydrogenase (11betaHSD) in the rat endolymphatic sac. Acta Otolaryngol 130:228–232. https://doi.org/10.3109/00016480903092357
Akiyama K, Miyashita T, Matsubara A, Mori N (2011) Specific RNA collection from the rat endolymphatic sac by laser-capture microdissection (LCM): LCM of a very small organ surrounded by bony tissues. Methods Mol Biol 755:441–448. https://doi.org/10.1007/978-1-61779-163-5_37
Akiyama K, Miyashita T, Mori T, Inamoto R, Mori N (2008) Expression of thiazide-sensitive Na+-Cl- cotransporter in the rat endolymphatic sac. Biochem Biophys Res Commun 371:649–653. https://doi.org/10.1016/j.bbrc.2008.04.081
Bagger-Sjoback D, Rask-Andersen H (1986) The permeability barrier of the endolymphatic sac. A hypothesis of fluid and electrolyte exchange based on freeze fracturing. Am J Otol 7:134–140
Basura GJ, Adams ME, Monfared A, Schwartz SR, Antonelli PJ, Burkard R, Bush ML, Bykowski J, Colandrea M, Derebery J, Kelly EA, Kerber KA, Koopman CF, Kuch AA, Marcolini E, McKinnon BJ, Ruckenstein MJ, Valenzuela CV, Vosooney A, Walsh SA, Nnacheta LC, Dhepyasuwan N, Buchanan EM (2020) Clinical practice guideline: Meniere’s disease. Otolaryngol Head Neck Surg 162:S1–S55. https://doi.org/10.1177/0194599820909438
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. https://doi.org/10.1373/clinchem.2008.112797
Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC (1994) Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367:463–467. https://doi.org/10.1038/367463a0
Chen J, Ruan R (2009) Identifying stable reference genes for evaluation of antioxidative enzyme gene expression in auditory cortex and cochlea of young and old Fischer 344 rats. Acta Otolaryngol 129:644–650. https://doi.org/10.1080/00016480802311015
Dahlmann A, von During M (1995) The endolymphatic duct and sac of the rat: a histological, ultrastructural, and immunocytochemical investigation. Cell Tissue Res 282:277–289
Devaiah AK, Ator GA (2000) Clinical indicators useful in predicting response to the medical management of Meniere’s disease. Laryngoscope 110:1861–1865. https://doi.org/10.1097/00005537-200011000-00018
Eckhard AH, Zhu M, O’Malley JT, Williams GH, Loffing J, Rauch SD, Nadol JB Jr, Liberman MC, Adams JC (2019) Inner ear pathologies impair sodium-regulated ion transport in Meniere’s disease. Acta Neuropathol 137:343–357. https://doi.org/10.1007/s00401-018-1927-7
Furuta H, Sato C, Kawaguchi Y, Miyashita T, Mori N (1999) Expression of mRNAs encoding hormone receptors in the endolymphatic sac of the rat. Acta Otolaryngol 119:53–57
Guild S (1927) Observations upon the structure and normal contents of the ductus and saccus endolymphaticus in the guinea-pig (Cavia cobaya). Am J Anat 39:1–56. https://doi.org/10.1002/aja.1000390102
He FJ, Li J, Macgregor GA (2013) Effect of longer term modest salt reduction on blood pressure: cochrane systematic review and meta-analysis of randomised trials. BMJ 346:f1325. https://doi.org/10.1136/bmj.f1325
Huggett J, Dheda K, Bustin S, Zumla A (2005) Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 6:279–284. https://doi.org/10.1038/sj.gene.6364190
Kim SH, Nam GS, Choi JY (2019) Pathophysiologic findings in the human endolymphatic sac in endolymphatic hydrops: functional and molecular evidence. Ann Otol Rhinol Laryngol 128:76S-83S. https://doi.org/10.1177/0003489419837993
Kimura Y, Kubo S, Koda H, Shigemoto K, Sawabe M, Kitamura K (2013) RNA analysis of inner ear cells from formalin fixed paraffin embedded (FFPE) archival human temporal bone section using laser microdissection–a technical report. Hear Res 302:26–31. https://doi.org/10.1016/j.heares.2013.04.008
Lewis F, Maughan NJ, Smith V, Hillan K, Quirke P (2001) Unlocking the archive–gene expression in paraffin-embedded tissue. J Pathol 195:66–71. https://doi.org/10.1002/1096-9896(200109)195:1<66::AID-PATH921>3.0.CO;2-F
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Masuda N, Ohnishi T, Kawamoto S, Monden M, Okubo K (1999) Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res 27:4436–4443
Matsubara A, Miyashita T, Inamoto R, Hoshikawa H, Mori N (2014) Cystic fibrosis transmembrane conductance regulator in the endolymphatic sac of the rat. Auris Nasus Larynx 41:409–412. https://doi.org/10.1016/j.anl.2014.02.005
Melgar-Rojas P, Alvarado JC, Fuentes-Santamaria V, Gabaldon-Ull MC, Juiz JM (2015) Validation of reference genes for RT-qPCR analysis in noise-induced hearing loss: a study in wistar rat. PLoS ONE 10:e0138027. https://doi.org/10.1371/journal.pone.0138027
Miyashita T, Inamoto R, Fukuda S, Hoshikawa H, Hitomi H, Kiyomoto H, Nishiyama A, Mori N (2017) Hormonal changes following a low-salt diet in patients with Meniere’s disease. Auris Nasus Larynx 44:52–57. https://doi.org/10.1016/j.anl.2016.03.001
Miyashita T, Tatsumi H, Hayakawa K, Mori N, Sokabe M (2007) Large Na(+) influx and high Na(+), K (+)-ATPase activity in mitochondria-rich epithelial cells of the inner ear endolymphatic sac. Pflugers Arch 453:905–913. https://doi.org/10.1007/s00424-006-0166-2
Moller MN, Kirkeby S, Vikesa J, Nielsen FC, Caye-Thomasen P (2015) Gene expression in the human endolymphatic sac: the solute carrier molecules in endolymphatic fluid homeostasis. Otol Neurotol 36:915–922. https://doi.org/10.1097/MAO.0000000000000669
Mori N, Miyashita T, Inamoto R, Matsubara A, Mori T, Akiyama K, Hoshikawa H (2017) Ion transport its regulation in the endolymphatic sac: suggestions for clinical aspects of Meniere’s disease. Eur Arch Otorhinolaryngol 274:1813–1820. https://doi.org/10.1007/s00405-016-4362-1
Mori N, Uozumi N, Yura K, Sakai S (1990) The difference in endocochlear and endolymphatic sac d.c. potentials in response to furosemide and canrenoate as diuretics. Eur Arch Otorhinolaryngol 247:371–373
Mori N, Wu D (1996) Low-amiloride-affinity Na+ channel in the epithelial cells isolated from the endolymphatic sac of guinea-pigs. Pflugers Arch 433:58–64
Murillo-de-Ozores AR, Rodriguez-Gama A, Carbajal-Contreras H, Gamba G, Castaneda-Bueno M (2021) WNK4 kinase: from structure to physiology. Am J Physiol Renal Physiol 320:F378–F403. https://doi.org/10.1152/ajprenal.00634.2020
Sharon JD, Trevino C, Schubert MC, Carey JP (2015) Treatment of Meniere’s disease. Curr Treat Options Neurol 17:341. https://doi.org/10.1007/s11940-015-0341-x
Stankovic KM, Corfas G (2003) Real-time quantitative RT-PCR for low-abundance transcripts in the inner ear: analysis of neurotrophic factor expression. Hear Res 185:97–108
Valinsky WC, Touyz RM, Shrier A (2018) Aldosterone, SGK1, and ion channels in the kidney. Clin Sci (Lond) 132:173–183. https://doi.org/10.1042/CS20171525
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Verouti SN, Boscardin E, Hummler E, Frateschi S (2015) Regulation of blood pressure and renal function by NCC and ENaC: lessons from genetically engineered mice. Curr Opin Pharmacol 21:60–72. https://doi.org/10.1016/j.coph.2014.12.012
Vikhe Patil K, Canlon B, Cederroth CR (2015) High quality RNA extraction of the mammalian cochlea for qRT-PCR and transcriptome analyses. Hear Res 325:42–48. https://doi.org/10.1016/j.heares.2015.03.008
Funding
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (#21K09634 to TM).
Author information
Authors and Affiliations
Contributions
AM, TM, HH, and NM designed the study. AM, TM, and KN performed the experiments. KN and SYS contributed reagents and analytical tools. AM, TM, and KN analyzed the data. AM, TM, KN, and SYS wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The animal protocols used were approved by the Animal Care and Use Committee of Kagawa University. All experiments were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsubara, A., Miyashita, T., Nakashima, K. et al. Low-salt diet increases mRNA expression of aldosterone-regulated transporters in the intermediate portion of the endolymphatic sac. Pflugers Arch - Eur J Physiol 474, 505–515 (2022). https://doi.org/10.1007/s00424-021-02661-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-021-02661-9