Abstract
Ion transport and its regulation in the endolymphatic sac (ES) are reviewed on the basis of recent lines of evidence. The morphological and physiological findings demonstrate that epithelial cells in the intermediate portion of the ES are more functional in ion transport than those in the other portions. Several ion channels, ion transporters, ion exchangers, and so on have been reported to be present in epithelial cells of ES intermediate portion. An imaging study has shown that mitochondria-rich cells in the ES intermediate portion have a higher activity of Na+, K+-ATPase and a higher Na+ permeability than other type of cells, implying that molecules related to Na+ transport, such as epithelial sodium channel (ENaC), Na+–K+–2Cl− cotransporter 2 (NKCC2) and thiazide-sensitive Na+–Cl− cotransporter (NCC), may be present in mitochondria-rich cells. Accumulated lines of evidence suggests that Na+ transport is most important in the ES, and that mitochondria-rich cells play crucial roles in Na+ transport in the ES. Several lines of evidence support the hypothesis that aldosterone may regulate Na+ transport in ES, resulting in endolymph volume regulation. The presence of molecules related to acid/base transport, such as H+-ATPase, Na+–H+ exchanger (NHE), pendrin (SLC26A4), Cl−–HCO3 − exchanger (SLC4A2), and carbonic anhydrase in ES epithelial cells, suggests that acid/base transport is another important one in the ES. Recent basic and clinical studies suggest that aldosterone may be involved in the effect of salt-reduced diet treatment in Meniere’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endolymphatic system homeostasis is crucial to maintain the normal function in the inner ear [1]. The stria vascularis in the cochlea, the dark cells in the vestibular organ, and the endolymphatic sac (ES) are mainly involved in the maintenance of homeostasis in the endolymphatic system [1]. Two main homeostatic mechanisms of inner ear fluid regulation have been proposed, i.e., radial and longitudinal endolymph movements [2, 3]. The physiology of the stria vascularis has been clarified on the basis of more studies, whereas the ES physiology is still unknown in many parts because of less research.
One of the pathological findings caused by the disturbance of homeostasis in the endolymphatic system is endolymphatic hydrops, which is known to be the typical pathological finding of Menière’s disease [4, 5]. The obliteration of the endolymphatic sac and endolymphatic duct induces endolymphatic hydrops in experimental animals [6]. Therefore, the ES is assumed to play crucial roles in maintaining the endolymphatic system homeostasis. It is important to know the roles of the ES in the endolymphatic system homeostasis to elucidate the pathogenesis underlying the development of Menière’s disease. Recent research on the ES has revealed the aspects of ion transport in the ES. The present review will outline ion transport and its regulation in the ES on the basis of recent research findings with suggestions for clinical aspects of Menière’s disease from the viewpoint of ion transport in the ES.
Morphology of ES and classification of ES epithelial cells
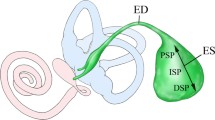
The ES is divided into the following three parts on the basis of morphological features: proximal, intermediate, and distal portions [2, 7]. The morphological findings imply that epithelial cells in the intermediate portion may be more functional in ion transport than those in the other portions [2, 8]. The epithelial cells in the intermediate portion have been recently classified electronmicroscopically into two types of cells: mitochondria-rich cells and ribosome-rich cells in the rat [8]. Mitochondria-rich cells have been reported to occupy 20–25% of epithelial cells in the intermediate portion of the rat [8]. Cytoorganelle-rich cells and filament-rich cells reported in the guinea pig [7] and the mouse [9] correspond to mitochondria-rich cells and ribosome-rich cells in the rat, respectively. Mitochondria-rich cells and ribosome-rich cells correspond roughly to light cells and dark cells termed by Lundquist [2], repectively. However, it has been pointed out that both cytoorganelle-rich cells and filament-rich cells in the guinea pig and the mouse [9] and both mitochondria-rich cells and ribosome-rich cells in the rat [8] may be stained lightly or darkly as fixation artifacts by electron microscope. Terms of mitochondria-rich cell and ribosome-rich cell have been widely used [10–12]. Table 1 summarizes the classification of epithelial cells in several species based on the morphological findings [2, 7–9, 13–15].
Resting potential and ion concentration in ES endolymph
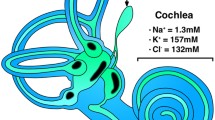
Endolymph in the ES is quite different in resting potential, ion concentration and pH from that in the other parts of the inner ear, such as the cochlea or vestibular organ (Table 2) [16–27]. It should be stressed that resting potential in the ES is higher than that in endolymph of the vestibular organ, although it is quite lower than that in the cochlea, and that endolymph in the ES has higher Na+ and lower K+ and Cl− concentrations and lower pH than cochlear and vestibular endolymph.
Resting potential in the ES named endolymphatic sac potential (ESP) [28, 29] was found by Amano et al. [20]. ESP, which is oxygen-dependent, has the following different properties from resting potentials in cochlear and vestibular endolymph:
- 1.
-
2.
ESP shows different responses to several diuretics from resting potentials in cochlear and vestibular endolymph [28, 31–34]. It is less sensitive to loop diuretics, whereas it is more sensitive to canrenoate, an aldosterone antagonist, and acetazolamide, a carbonic anhydrase inhibitor.
-
3.
Catecholamines produce a reversible depression in ESP by β2 adrenergic action [35].
-
4.
ESP is mainly composed of an acetazolamide-sensitive part and an isoproterenol-sensitive part [36].
ESP is assumed to have plural origins, one of which may be H+-ATPase [37]. The presence of ESP may prompt Na+ transport from the ES lumen to the outside although its physiological roles remain to be clarified.
Molecules related to transport of ion and water in ES epithelial cells
Molecules related to ion transport in epithelial cells in the intermediate portion of the ES are shown in Table 3 [10, 38–60]. The type of cells with most molecules has not been identified except only a few molecules [10, 46]. It should be stressed that Na+–K+–2Cl− cotransporter 2 (NKCC2) [56, 57, 59, 60] and thiazide-sensitive Na+–Cl− cotransporter (NCC) [55, 58], which had been previously recognized to be selectively located in kidney, are present in ES epithelial cells.
Several isoforms of aquaporin (AQP) as molecules related to water transport in ES epithelial cells have been reported, as shown in Table 3 [44, 57, 59, 61–63].
Electrophysiological profile on ion transport in ES epithelial cells
Recent lines of evidence on ion transport in the ES (including cation, anion and acid/base transports) is as follows:
-
1.
ES epithelial cells have resting membrane potential of approximately −60 mV [41].
-
2.
ES endolymph has resting potential of approximately +15 mV [24, 37, 64].
-
3.
ES endolymph has a higher Na+ concentration and lower K+ and Cl− concentrations. There are active Na+ and Cl− outflows from the ES lumen into the outside [20, 24].
-
4.
K+ and Na+ are permeable ions, but Cl− is a negligible ion in the ES isolated epithelial cells [65].
-
5.
Mitochondria-rich cells in the ES have a higher activity of Na+, K+-ATPase and a higher Na+ permeability [11].
-
6.
ES endolymph has a weak acidity [26], in which H+-ATPase may be involved [66].
-
7.
ES epithelial cells have molecules related to acid/base transport, such as H+-ATPase [49, 50], Na+–H+ exchanger (NHE) [54, 55], pendrin (SLC26A4) [10, 50, 55], Cl−–HCO3 − exchanger (SLC4A2) [49], and carbonic anhydrase [50–53].
Ion transport properties in ES epithelial cells
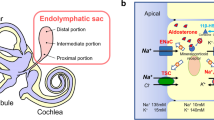
Our Na+ imaging study [11] demonstrates that mitochondria-rich cells in the ES have a higher activity of Na+, K+-ATPase and a higher Na+ permeability, strongly suggesting that molecules related to Na+ transport may be densely located in mitochondria-rich cells. Mitochondria-rich cells in ES have characteristic qualities of Na+ absorption (Fig. 1). Na+ enters the cell across the apical membrane through ion channels and ion transporters driven by an estimated electrochemical driving force of approximately 140 mV. K+ enters the cell from endolymph across the apical membrane through the non-selective cation channel driven by an estimated electrochemical driving force of approximately 20 mV and through NKCC2 driven by a higher Na+ inflow. Na+ is removed across the basolateral membrane by Na+, K+-ATPase. K+ is brought into the cell by the pump, and subsequently diffuses out through K+ channel (outward delayed rectifier), which is involved in the maintenance of negative intracellular potential. The model is similar to that found classically in several other Na+-absorbing epithelia [67]. Na+ transport is a major part of ion transport system in ES.
Na+ and K+ transport model in mitochondria-rich epithelial cells of the endolymphatic sac. Large positive electrochemical gradients for Na+ promote Na+ inflow into the cell from apical and basolateral membrane. Inflowing Na+ is actively transported by Na+, K+-ATPase with a high activity. Na+ absorption is followed by water movement from endolymph to the outside
Molecules related to acid/base transport are H+-ATPase, Na+–H+ exchanger (NHE) and pendrin in the apical membrane, Cl−–HCO3 − exchanger (SLC4A2) in the basolateral membrane, and intracellular and membrane-bound carbonic anhydrase [50]. H+-ATPase, pendrin, and carbonic anhydrase have been shown to be localized in the same type of ES epithelial cells [50]. Pendrin has been reported to be present in mitochondria-rich cells [10]. For the maintenance of acidity in ES lumen, the inflow of H+ into the lumen is necessary to be larger than the inflow of HCO3 −. NHE in the apical membrane, which is presumed to be active due to a large Na+ inflow into the cell, besides H+-ATPase may be largely involved in the acidity of ES endolymph. Acid–base transport is another important part of ion transport system in ES.
Molecules related to Cl− transport have been reported to be an ion channel (cystic fibrosis transmembrane conductance regulator, CFTR) in the apical membrane [40], ion cotransporters in the apical membrane (NKCC2 and NCC) [56, 58], and ion exchangers (pendrin in the apical membrane [50], and Cl−–HCO3 − exchanger (SLC4A2) in the basolateral membrane [49]). CFTR has been shown to be co-localized with ENaC [40]. There has been no report on intracellular Cl− concentration of ES epithelial cells. When intracellular Cl− concentration of ES epithelial cells is assumed to be similar to 4 mM in other tissues [68], the electrochemical gradient for Cl− into the cell is calculated to be around 0 mV. Since the results using whole cell patch clamp showed that Cl− current was negligible in the ES isolated epithelial cells [65], it is unlikely that Cl− is a leading ion in ES ion transport system. Cl− is assumed to be transported following Na+ transport and acid/base transport.
Regulation of Na+ transport in ES
Several agents, such as vasopressin [44, 69, 70], aldosterone [71], cortisol [72, 73], atrial natriuretic peptide [74], catecholamines [75–77], and ATP [78], have been suggested as the candidates for regulators of ion transport in ES. It has been known that several hormones such as vasopressin and aldosterone regulate Na+ transport in other tissues such as the kidney [79, 80, 81]. More lines of evidence supporting aldosterone as a regulator of Na+ transport in ES has been accumulated in comparison with other candidates as follows:
-
1.
The presence of mineralocorticoid receptors (MRs) in ES epithelial cells has been shown [71].
-
2.
11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which enables aldosterone selectively to bind to MRs by converting cortisol (corticosterone) into inactive metabolites, has been detected in ES epithelial cells [82]. The presence of 11β-HSD2 is considered essential in aldosterone-target tissues [83]. The absence of 11β-HSD2 has been shown in cochlear and vestibular tissues [84].
-
3.
The presence of ENaC in ES epithelial cell has been shown [40].
-
4.
The presence of NCC in ES epithelial cells has been shown [58]. NCC had been accepted to have a specific localization in distal convoluted tubule of the kidney until NCC was found in ES epithelial cells. NCC is regulated by aldosterone [85].
-
5.
The antagonist of aldosterone, canrenoate, intravenously produced a decreased ESP change with no change in the endocochlear potential, suggesting that aldosterone could act more sensitively on the ES [28, 31].
Aldosterone activates Na+ transport from endolymph into ES epithelial cells, mainly mitochondria-rich cells in a similar manner to epithelial cells in other aldosterone-target tissues (Fig. 2). Activation of Na+ absorption increases water absorption, resulting in increased endolymph absorption.
Several AQP isoforms including AQP2 activated by vasopressin have been detected in the ES epithelial cells, as shown in Table 3. The specific presence of various kinds of AQP isoforms in the ES reinforces more effective water movement accompanying Na+ transport, resulting in efficient endolymph absortption in the ES.
Suggestions for clinical aspects of Meniere’s disease from recent evidence on ion transport in ES
Plasma aldosterone concentration has been reported to be within the normal range in patients with Meniere’s disease [86, 87]. Therefore, the elevation of plasma aldosterone concentration is not considered to be directly involved in the pathogenesis of Meniere’s disease. However, the findings suggesting that aldosterone may be involved in endolymph volume regulation through the regulation of Na+ transport in the ES give an experimental support to empirical salt-reduced diet treatment in Meniere’s disease. Our recent study [87] has shown that salt-reduced diet with no administration of thiazide is an effective treatment in Meniere’s disease, and that during 2-year treatment period, salt-reduced diet induced the elevation of plasma aldosterone concentration with no change in other hormones, such as vasopressin, cortisol, and brain natriuretic peptide in patients with Meniere’s disease as the elevation of plasma aldosterone concentration has been reported in patients with hypertension [88]. The presence of NCC in the ES besides the kidney [55, 58] may propose a necessity to reconsider the indication of thiazide in Meniere’s disease.
The presence of vasopressin-AQP-2 system in ES epithelia may play important roles in endolymph volume regulation [44, 61], suggesting that vasopressin-AQP-2 system in the ES would be involved in the development of Meniere’s disease [70, 89, 90].
The findings that catecholamines increased the hydrostatic pressure of cochlear and vestibular endolymph [76, 77] probably through β adrenergic action on the ES give a basic support to the clinical empirical finding that the stress often worsens the symptoms in patients with Meniere’s disease. Results that the degree of an increase in endolymphatic hydrostatic pressure induced by β agonist was significantly larger in the pars inferior than that in the pars superior [77] may give any suggestions in considering the clinical course in Meniere’s disease.
References
Sterkers O, Ferrary E, Amiel C (1988) Production of inner ear fluids. Physiol Rev 68(4):1083–1128
Lundquist PG (1965) The endolymphatic duct and sac in the guinea pig. An electron microscope and experimental investigation. Acta Otolaryngol (Stockh) Suppl 201:1–108
Eckhard A, Dos Santos A, Liu W, Bassiouni M, Arnold H, Gleiser C, Hirt B, Harteneck C, Muller M, Rask-Andersen H, Lowenheim H (2015) Regulation of the perilymphatic-endolymphatic water shunt in the cochlea by membrane translocation of aquaporin-5. Pflugers Arch 467(12):2571–2588. doi:10.1007/s00424-015-1720-6
Yamakawa K (1938) Auditory organs in patient with Meniere’s syndrome. J Otolaryngol Jpn 44:2310–2312
Hallpike CS, Cairns H (1938) Observation on the pathology of Meniere’s syndrome. J Laryngol Otol 53:625–655
Kimura RS, Schuknecht HF (1965) Membranous hydrops in the inner ear of the guinea pig after obliteration of the endolymphatic sac. Pract Otorhinolaryngol 27:343–354
Fukazawa K, Matsunaga T, Fujita H (1990) Ultrastructure of the endolymphatic sac in the guinea pig; with special regards to classification of cell types of the epithelium and uptake of india ink particles into free floating cells and epithelial cells of the sac. J Clin Electron Microsc 23:135–147
Dahlmann A, von During M (1995) The endolymphatic duct and sac of the rat: a histological, ultrastructural, and immunocytochemical investigation. Cell Tissue Res 282(2):277–289
Furuta H, Mori N, Fujita M, Sakai S (1991) Ultrastructure of the endolymphatic sac in the mouse. Acta Anat (Basel) 141(3):193–198
Royaux IE, Belyantseva IA, Wu T, Kachar B, Everett LA, Marcus DC, Green ED (2003) Localization and functional studies of pendrin in the mouse inner ear provide insight about the etiology of deafness in pendred syndrome. J Assoc Res Otolaryngol JARO 4(3):394–404
Miyashita T, Tatsumi H, Hayakawa K, Mori N, Sokabe M (2007) Large Na+ influx and high Na(+), K (+)-ATPase activity in mitochondria-rich epithelial cells of the inner ear endolymphatic sac. Pflugers Arch 453(6):905–913
Kim HM, Wangemann P (2010) Failure of fluid absorption in the endolymphatic sac initiates cochlear enlargement that leads to deafness in mice lacking pendrin expression. PLoS One 5(11):e14041. doi:10.1371/journal.pone.0014041
Friberg U (1985) The endolymphatic duct and sac: an ultrastructural and experimental investigation. University of Uppsala, Uppsala
Manni JJ (1987) The endolymphatic duct and sac of the rat: a histophysiological study. University of Nijmegen, Nijmegen
Lim DJ (1999) Ultrastructure of the endolymphatic duct and sac in normal and Meniere’s disease. Kugler Publications, The Hague
Johnstone BM, Sellick PM (1972) The peripheral auditory apparatus. Q Rev Biophys 5(1):1–57
Konishi T, Hamrick PE (1978) Ion transport in the cochlea of guinea pig II. Chloride transport. Acta Otolaryngol 86(3–4):176–184
Bosher SK (1979) The nature of the negative endocochlear potentials produced by anoxia and ethacrynic acid in the rat and guinea-pig. J Physiol 293:329–345
Morgenstern C, Amano H, Orsulakova A (1982) Ion transport in the endolymphatic space. Am J Otolaryngol 3(5):323–327
Amano H, Orsulakova A, Morgenstern C (1983) Intracellular and extracellular ion content of the endolymphatic sac. Arch Otorhinolaryngol 237(3):273–277
Konishi T, Hamrick PE, Mori H (1984) Water permeability of the endolymph–perilymph barrier in the guinea pig cochlea. Hear Res 15(1):51–58
Ninoyu O, Meyer zum Gottesberge AM (1986) Calcium transport in the endolymphatic space of cochlea and vestibular organ. Acta Otolaryngol 102(3–4):222–227
Ninoyu O, Morgenstern C (1986) Calcium transport in the endolymphatic sac. ORL J Otorhinolaryngol Relat Spec 48(4):199–202
Mori N, Ninoyu O, Morgenstern C (1987) Cation transport in the ampulla of the semicircular canal and in the endolymphatic sac. Arch Otorhinolaryngol 244(1):61–65
Salt AN, Inamura N, Thalmann R, Vora A (1989) Calcium gradients in inner ear endolymph. Am J Otolaryngol 10(6):371–375
Tsujikawa S, Yamashita T, Amano H, Kumazawa T, Vosteen KH (1992) Acidity in the endolymphatic sac fluid of guinea pigs. ORL J Otorhinolaryngol Relat Spec 54(4):198–200
Tsujikawa S, Yamashita T, Tomoda K, Iwai H, Kumazawa H, Cho H, Kumazawa T (1993) Effects of acetazolamide on acid-base balance in the endolymphatic sac of the guinea pig. Acta Otolaryngol Suppl 500:50–53
Mori N, Uozumi N, Yura K, Sakai S (1990) The difference in endocochlear and endolymphatic sac DC potentials in response to furosemide and canrenoate as diuretics. Eur Arch Otorhinolaryngol 247(6):371–373
Mori N, Uozumi N, Sakai S (1990) Catecholamines depress endolymphatic sac direct current potential in guinea pigs. Am J Physiol 259(5 Pt 2):R921–R924
Mori N, Uozumi N, Sakai S (1991) Response of the endolymphatic sac DC potential to asphyxia. Acta Otolaryngol 111(1):70–74
Mori N, Yura K, Uozumi N, Sakai S (1991) Effect of aldosterone antagonist on the DC potential in the endolymphatic sac. Ann Otol Rhinol Laryngol 100(1):72–75
Uozumi N, Mori N, Sakai S (1991) The effect of acetazolamide on the endolymphatic sac DC potential. Acta Otolaryngol 111(5):921–925
Kusakari J, Thalmann R (1976) Effects of anoxia and ethacrynic acid upon ampullar endolymphatic potential and upon high energy phosphates in ampullar wall. Laryngoscope 86(1):132–147. doi:10.1288/00005537-197601000-00025
Kusakari J, Ise I, Comegys TH, Thalmann I, Thalmann R (1978) Effect of ethacrynic acid, furosemide, and ouabain upon the endolymphatic potential and upon high energy phosphates of the stria vascularis. Laryngoscope 88(1 Pt 1):12–37
Mori N, Uozumi N (1991) Evidence that beta 2-receptors mediate action of catecholamines on endolymphatic sac DC potential. Am J Physiol 260(5 Pt 2):R911–R915
Mori N, Uozumi N (1992) Interaction of catecholamine and acetazolamide in the action on the endolymphatic sac direct current potential. Acta Otolaryngol 112(1):65–69
Couloigner V, Teixeira M, Hulin P, Sterkers O, Bichara M, Escoubet B, Planelles G, Ferrary E (2000) Effect of locally applied drugs on the pH of luminal fluid in the endolymphatic sac of guinea pig. Am J Physiol Regul Integr Comp Physiol 279(5):R1695–R1700
Mori N, Wu D (1996) Low-amiloride-affinity Na+ channel in the epithelial cells isolated from the endolymphatic sac of guinea-pigs. Pflugers Arch 433(1–2):58–64
Kim SH, Park HY, Choi HS, Chung HP, Choi JY (2009) Functional and molecular expression of epithelial sodium channels in cultured human endolymphatic sac epithelial cells. Otol Neurotol. doi:10.1097/MAO.0b013e31819a8e0e
Matsubara A, Miyashita T, Inamoto R, Hoshikawa H, Mori N (2014) Cystic fibrosis transmembrane conductance regulator in the endolymphatic sac of the rat. Auris Nasus Larynx 41(5):409–412. doi:10.1016/j.anl.2014.02.005
Wu D, Mori N (1996) Outward K+ current in epithelial cells isolated from intermediate portion of endolymphatic sac of guinea pigs. Am J Physiol 271(5 Pt 1):C1765–C1773
Wu D, Mori N (1999) Extracellular ATP-induced inward current in isolated epithelial cells of the endolymphatic sac. Biochim Biophys Acta 1419(1):33–42
Miyashita T, Tatsumi H, Furuta H, Mori N, Sokabe M (2001) Calcium-sensitive nonselective cation channel identified in the epithelial cells isolated from the endolymphatic sac of guinea pigs. J Membr Biol 182(2):113–122
Taguchi D, Takeda T, Kakigi A, Takumida M, Nishioka R, Kitano H (2007) Expressions of aquaporin-2, vasopressin type 2 receptor, transient receptor potential channel vanilloid (TRPV)1, and TRPV4 in the human endolymphatic sac. Laryngoscope 117(4):695–698. doi:10.1097/mlg.0b013e318031c802
Ishibashi T, Takumida M, Akagi N, Hirakawa K, Anniko M (2008) Expression of transient receptor potential vanilloid (TRPV) 1, 2, 3, and 4 in mouse inner ear. Acta Otolaryngol 128(12):1286–1293. doi:10.1080/00016480801938958
Kumagami H, Terakado M, Sainoo Y, Baba A, Fujiyama D, Fukuda T, Takasaki K, Takahashi H (2009) Expression of the osmotically responsive cationic channel TRPV4 in the endolymphatic sac. Audiol Neurootol 14(3):190–197. doi:10.1159/000180290
Kim SH, Kim BG, Kim JY, Roh KJ, Suh MJ, Jung J, Moon IS, Moon SK, Choi JY (2015) Electrogenic transport and K(+) ion channel expression by the human endolymphatic sac epithelium. Sci Rep 5:18110. doi:10.1038/srep18110
Mizukoshi F, Bagger-Sjoback D, Rask-Andersen H, Wersall J (1988) Cytochemical localization of Na-K ATPase in the guinea pig endolymphatic sac. Acta Otolaryngol 105(3–4):202–208
Stankovic KM, Brown D, Alper SL, Adams JC (1997) Localization of pH regulating proteins H+ ATPase and Cl−/HCO3 − exchanger in the guinea pig inner ear. Hear Res 114(1–2):21–34
Dou H, Xu J, Wang Z, Smith AN, Soleimani M, Karet FE, Greinwald JH Jr, Choo D (2004) Co-expression of pendrin, vacuolar H+-ATPase alpha4-subunit and carbonic anhydrase II in epithelial cells of the murine endolymphatic sac. J Histochem Cytochem Off J Histochem Soc 52(10):1377–1384. doi:10.1369/jhc.3A6228.2004
Lim DJ, Karabinas C, Trune DR (1983) Histochemical localization of carbonic anhydrase in the inner ear. Am J Otolaryngol 4(1):33–42
Takumida M, Bagger-Sjoback D, Rask-Andersen H (1988) Ultrastructural localization of carbonic anhydrase and its possible role in the endolymphatic sac. ORL J Otorhinolaryngol Relat Spec 50(3):170–175
Furuta H, Mori N, Sakai S (1993) Immunohistochemical localization of carbonic anhydrase in the endolymphatic sac of the mouse and guinea pig. ORL J Otorhinolaryngol Relat Spec 55(1):13–17
Wu D, Mori N (1998) Evidence for the presence of a Na+–H+ exchanger in the endolymphatic sac epithelium of guinea-pigs. Pflugers Arch 436(2):182–188
Moller MN, Kirkeby S, Vikesa J, Nielsen FC, Caye-Thomasen P (2015) Gene expression in the human endolymphatic sac: the solute carrier molecules in endolymphatic fluid homeostasis. Otol Neurotol 36(5):915–922. doi:10.1097/MAO.0000000000000669
Akiyama K, Miyashita T, Mori T, Mori N (2007) Expression of the Na-K-2Cl cotransporter in the rat endolymphatic sac. Biochem Biophys Res Commun 364:913–917
Kakigi A, Nishimura M, Takeda T, Taguchi D, Nishioka R (2009) Expression of aquaporin1, 3, and 4, NKCC1, and NKCC2 in the human endolymphatic sac. Auris Nasus Larynx 36(2):135–139. doi:10.1016/j.anl.2008.04.012
Akiyama K, Miyashita T, Mori T, Inamoto R, Mori N (2008) Expression of thiazide-sensitive Na+–Cl− cotransporter in the rat endolymphatic sac. Biochem Biophys Res Commun 371(4):649–653. doi:10.1016/j.bbrc.2008.04.081
Nishimura M, Kakigi A, Takeda T, Takeda S, Doi K (2009) Expression of aquaporins, vasopressin type 2 receptor, and Na+(-)K+(-)Cl(-) cotransporters in the rat endolymphatic sac. Acta Otolaryngol 129(8):812–818. doi:10.1080/00016480802441754
Akiyama K, Miyashita T, Matsubara A, Mori N (2010) The detailed localization pattern of Na+/K+/2Cl− cotransporter type 2 and its related ion transport system in the rat endolymphatic sac. J Histochem Cytochem Off J Histochem Soc 58(8):759–763. doi:10.1369/jhc.2010.956045
Beitz E, Kumagami H, Krippeit-Drews P, Ruppersberg JP, Schultz JE (1999) Expression pattern of aquaporin water channels in the inner ear of the rat. The molecular basis for a water regulation system in the endolymphatic sac. Hear Res 132(1–2):76–84
Couloigner V, Berrebi D, Teixeira M, Paris R, Florentin A, Bozorg Grayeli A, Cluzeaud F, Sterkers O, Peuchmaur M, Ferrary E (2004) Aquaporin-2 in the human endolymphatic sac. Acta Otolaryngol 124(4):449–453
Takumida M, Kakigi A, Egami N, Nishioka R, Anniko M (2012) Localization of aquaporins 1, 2, and 3 and vasopressin type 2 receptor in the mouse inner ear. Acta Otolaryngol 132(8):807–813. doi:10.3109/00016489.2012.662718
Mori N, Uozumi N (1991) Properties of the endolymphatic sac DC potential. Otol Jpn 1(Suppl 1):23–26
Mori N, Wu D, Furuta H (1998) Membrane potential in isolated epithelial cells of the endolymphatic sac in the guinea-pig. Acta Otolaryngol 118(2):192–197
Couloigner V, Loiseau A, Sterkers O, Amiel C, Ferrary E (1998) Effect of locally applied drugs on the endolymphatic sac potential. Laryngoscope 108(4 Pt 1):592–598
Miyashita T, Akiyama K, Inamoto R, Matsubara A, Nakagawa T, Yamaguchi F, Tokuda M, Mori N (2012) Presence of FXYD6 in the endolymphatic sac epithelia. Neurosci Lett 513(1):47–50. doi:10.1016/j.neulet.2012.02.005
Lodish HBA, Zipursky SL et al (2000) Molecular cell biology, 4th edn. W.H.Freeman and Company, New York
Kumagami H, Loewenheim H, Beitz E, Wild K, Schwartz H, Yamashita K, Schultz J, Paysan J, Zenner HP, Ruppersberg JP (1998) The effect of anti-diuretic hormone on the endolymphatic sac of the inner ear. Pflugers Arch 436(6):970–975
Kitahara T, Doi K, Maekawa C, Kizawa K, Horii A, Kubo T, Kiyama H (2008) Meniere’s attacks occur in the inner ear with excessive vasopressin type-2 receptors. J Neuroendocrinol 20(12):1295–1300
Furuta H, Sato C, Kawaguchi Y, Miyashita T, Mori N (1999) Expression of mRNAs encoding hormone receptors in the endolymphatic sac of the rat. Acta Otolaryngol 119(1):53–57
Shimazaki T, Ichimiya I, Suzuki M, Mogi G (2002) Localization of glucocorticoid receptors in the murine inner ear. Ann Otol Rhinol Laryngol 111(12 Pt 1):1133–1138
Aoki M, Wakaoka Y, Hayashi H, Nishihori T, Kuze B, Mizuta K, Ito Y (2011) The relevance of hypothalamus–pituitary–adrenocortical axis-related hormones to the cochlear symptoms in Meniere’s disease. Int J Audiol 50(12):897–904. doi:10.3109/14992027.2011.605807
Dornhoffer JL, Danner C, Zhou L, Li S (2002) Atrial natriuretic peptide receptor upregulation in the rat inner ear. Ann Otol Rhinol Laryngol 111(11):1040–1044
Matsubara A, Miyashita T, Inamoto R, Mori N (2013) Presence of adrenergic receptors in rat endolymphatic sac epithelial cells. J Membr Biol 246(2):109–114. doi:10.1007/s00232-012-9508-5
Inamoto R, Miyashita T, Akiyama K, Mori T, Mori N (2009) Endolymphatic sac is involved in the regulation of hydrostatic pressure of cochlear endolymph. Am J Physiol Regul Integr Comp Physiol 297(5):R1610–R1614. doi:10.1152/ajpregu.00073.2009
Inamoto R, Miyashita T, Matsubara A, Hoshikawa H, Mori N (2016) The difference in endolymphatic hydrostatic pressure elevation induced by isoproterenol between the ampulla and the cochlea. Auris Nasus Larynx. doi:10.1016/j.anl.2016.07.018
Mori T, Miyashita T, Akiyama K, Inamoto R, Mori N (2009) The expression of P2Y1, 2, 4, and 6 receptors in rat endolymphatic sac epithelia. Neuroreport 20(4):419–423. doi:10.1097/WNR.0b013e328325a926
Kortenoeven ML, Pedersen NB, Rosenbaek LL, Fenton RA (2015) Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am J Physiol Renal Physiol 309(4):F280–F299. doi:10.1152/ajprenal.00093.2015
Staruschenko A (2012) Regulation of transport in the connecting tubule and cortical collecting duct. Compr Physiol 2(2):1541–1584. doi:10.1002/cphy.c110052
Furuta H, Mori N, Sato C, Hoshikawa H, Sakai S, Iwakura S, Doi K (1994) Mineralocorticoid type I receptor in the rat cochlea: mRNA identification by polymerase chain reaction (PCR) and in situ hybridization. Hear Res 78(2):175–180
Akiyama K, Miyashita T, Matsubara A, Inamoto R, Mori T, Nishiyama A, Mori N (2010) Expression and localization of 11beta-hydroxysteroid dehydrogenase (11betaHSD) in the rat endolymphatic sac. Acta Otolaryngol 130:228–232. doi:10.1080/00016480903092357
Le Menuet D, Viengchareun S, Muffat-Joly M, Zennaro MC, Lombes M (2004) Expression and function of the human mineralocorticoid receptor: lessons from transgenic mouse models. Mol Cell Endocrinol 217(1–2):127–136
Kumagami H, Terakado M, Takahashi H (2013) Distribution of glucocorticoid receptors and 11beta-hydroxysteroid dehydrogenase isoforms in the human inner ear. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol 34(1):151–157. doi:10.1097/MAO.0b013e31826a55ad
Lou Y, Zhang F, Luo Y, Wang L, Huang S, Jin F (2016) Serum and glucocorticoid regulated kinase 1 in sodium homeostasis. Int J Mol Sci. doi:10.3390/ijms17081307
Mateijsen DJ, Kingma CM, De Jong PE, Wit HP, Albers FW (2001) Aldosterone assessment in patients with Meniere’s disease. ORL J Otorhinolaryngol Relat Spec 63(5):280–286
Miyashita T, Inamoto R, Fukuda S, Hoshikawa H, Hitomi H, Kiyomoto H, Nishiyama A, Mori N (2016) Hormonal changes following a low-salt diet in patients with Meniere’s disease. Auris Nasus Larynx. doi:10.1016/j.anl.2016.03.001
He FJ, Li J, Macgregor GA (2013) Effect of longer term modest salt reduction on blood pressure: cochrane systematic review and meta-analysis of randomised trials. BMJ (Clinical research ed) 346:f1325. doi:10.1136/bmj.f1325
Takeda T, Takeda S, Kakigi A, Okada T, Nishioka R, Taguchi D, Nishimura M, Nakatani H (2010) Hormonal aspects of Meniere’s disease on the basis of clinical and experimental studies. ORL J Otorhinolaryngol Relat Spec 71(Suppl 1):1–9. doi:10.1159/000265113
Steinbach S, Hundt W, Hamann KF, Werner JA, Mandic R (2012) Effect of thirst challenge on ADH levels in patients with bilateral Meniere’s disease. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc 120(7):405–409. doi:10.1055/s-0032-1309005
Acknowledgements
This study was supported by Grant Nos. 16591711 and 19791208 for Scientific Research from the Ministry of Education, Science, and Culture, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors do not have any conflicts.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mori, N., Miyashita, T., Inamoto, R. et al. Ion transport its regulation in the endolymphatic sac: suggestions for clinical aspects of Meniere’s disease. Eur Arch Otorhinolaryngol 274, 1813–1820 (2017). https://doi.org/10.1007/s00405-016-4362-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4362-1