Abstract
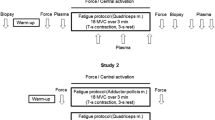
The effect of temperature on skeletal muscle ATP turnover, pulmonary oxygen uptake and single fibre ATP and PCr content was studied during intense cycling exercise. Six healthy male subjects performed 6-min intense (Δ50%LT-VO2peak) cycling, at 60 rpm, under conditions of normal (N) and elevated muscle temperature (ET). Muscle biopsies obtained from the vastus lateralis at rest, 2 and 6 min were analysed for homogenate ATP, PCr, lactate and glycogen, allowing estimation of anaerobic ATP turnover. Freeze-dried single fibres from biopsies were characterised according to their myosin heavy chain composition (type I, IIA or IIAX) and analysed for ATP and PCr content. Pulmonary gas exchange was measured throughout. There was no difference in pulmonary oxygen uptake between the trials. The elevation of muscle temperature resulted in a lower (P < 0.05) PCr content, higher (P < 0.05) lactate content and greater (P < 0.05) anaerobic ATP turnover after 2 min of exercise. There was no effect of temperature on these measures at 6 min. In single fibres it was observed that in ET, there was a lower (P < 0.05) PCr content in type I fibres after 2 min with no differences between conditions after 6 min. The present study demonstrates that elevation of muscle temperature results in a greater anaerobic ATP turnover and type I fibre PCr degradation during the initial 2 min of intense exercise.


Similar content being viewed by others
References
Asmussen E, Boje O (1945) Body temperature and the capacity for work. Acta Physiol Scand 10:1–22
Bangsbo J, Gollink PD, Graham TE, Juel C, Kiens B, Mizuno M, Saltin B (1990) Anaerobic energy production and O2 deficit–debt relationship during exhaustive exercise in humans. J Physiol 422:539–559
Beaver WL, Wasserman K, Whipp BJ (1985) Improved detection of lactate threshold during exercise using a log–log transformation. J Appl Physiol 59:1936–1940
Bell MP, Ferguson RA (2009) Interaction between muscle temperature and contraction velocity affects mechanical efficiency during moderate-intensity cycling exercise in young and older women. Journal of Applied Physiology 107:763–769
Beltman JG, de Haan A, Haan H, Gerrits HL, van Mechelen W, Sargeant AJ (2004) Metabolically assessed muscle fibre recruitment in brief isometric contractions at different intensities. Eur J Appl Physiol 92:485–492
Beltman JG, Sargeant AJ, Haan H, van Mechelen W, de Haan A (2004) Changes in PCr/Cr ratio on single characterized muscle fibre fragments after only a few maximal voluntary contractions in humans. Acta Physiol Scand 180:187–193
Bennett AF (1984) Thermal dependence of muscle function. Am J Physiol 247:R217–R229
Bergstrom J (1962) Muscle electrolytes in man. Scand J Clin Lab Invest suppl 68:1–101
Burnley M, Doust JH, Ball D, Jones AM (2002) Effects of prior heavy exercise on Vo2 kinetics during heavy exercise are related to changes in muscle activity. J Appl Physiol 93:167–174
Burnley M, Doust JH, Jones AM (2002) Effects of prior heavy exercise, prior sprint exercise and passive warming on oxygen uptake kinetics during heavy exercise in humans. Eur J Appl Physiol 87:424–432
Conjard A, Pette D (1999) Phosphocreatine as a marker of contractile activity in single muscle fibres. Pflugers Archiv 438:278–282
di Prampero PE, Ferretti G (1999) The energetics of anaerobic muscle metabolism: a reappraisal of older and recent concepts. Respiration Physiology 118:103–115
Edwards RHT, Harris RC, Hultman E, Kaijser L, Koh D, Nordesjo L-O (1972) Effect of temperature on muscle energy metabolism and endurance during successive isometric contractions, sustained to fatigue, of the quadriceps muscle in man. J Physiol 220:335–352
Fauteck SP, Kandarian SC (1995) Sensitive detection of myosin heavy chain composition in skeletal muscle under different loading conditions. Am J Physiol 268:C419–C424
Febbraio MA (2000) Does muscle function and metabolism affect exercise performance in the heat? Exerc Sport Sci Rev 28:171–176
Febbraio MA, Carey MF, Snow RJ, Stathis CG, Hargreaves M (1996) Influence of elevated muscle temperature on metabolism during intense, dynamic exercise. Am J Physiol 271:1251–1255
Fenn WO (1923) A quantitative comparison between the energy liberated and the work performed by the isolated sartorius muscle of the frog. J Physiol 58:175–203
Ferguson RA, Ball D, Sargeant AJ (2002) Effect of muscle temperature on rate of oxygen uptake during exercise in humans at different contraction frequencies. J Exp Biol 205:981–987
Ferguson RA, Krustrup P, Kjaer M, Mohr M, Ball D, Bangsbo J (2006) Effect of temperature on skeletal muscle energy turnover during dynamic knee-extensor exercise in humans. J Appl Physiol 101:47–52
Gladden LB (2000) Muscle as a consumer of lactate. Med Sci Sports Exerc 32:764–771
Gladden LB (2004) Lactate metabolism: a new paradigm for the third millennium. J Physiol 558:5–30
Gollnick PD, Armstrong RB, Saubert CW, Piehl K, Saltin B (1972) Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. Journal of Applied Physiology 33:312–319
Gray SR, De Vito G, Nimmo MA, Farina D, Ferguson RA (2006) Skeletal muscle ATP turnover and muscle fiber conduction velocity are elevated at higher muscle temperatures during maximal power output development in humans. Am J Physiol 290:R376–R382
Gray SR, Soderlund K, Ferguson RA (2008) ATP and phosphocreatine utilization in single human muscle fibres during the development of maximal power output at elevated muscle temperatures. J Sports Sci 26:701–707
Greenhaff PL, Nevill AM, Söderlund K, Bodin K, Boobis LH, Williams C, Hultman E (1994) The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol 478:149–155
Harris RC, Hultman E, Nordesjö L-O (1974) Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33:109–120
He Z-H, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C (2000) ATP consumption and efficiency of human single muscle fibres with different myosin isoform composition. Biophysical Journal 79:945–961
Infante AA, Klaupiks D, Davies RE (1965) Phosphorylcreatine consumption during single-working contractions of isolated muscle. Biochim Biophys Acta 94:504–515
Jones. AM (1998) A five year physiological case study of an olympic runner. Br J Sp Med 32:39–43
Karatzaferi C, Chinn MK, Cooke R (2004) The force exerted by a muscle cross-bridge depends directly on the strength of the actomyosin bond. Biophysical Journal 87:2532–2544
Koga S, Shiojiri T, Kondo N, Barstow TJ (1997) Effect of increased muscle temperature on oxygen uptake kinetics during exercise. J Appl Physiol 83:1333–1338
Larsson L, Moss RL (1993) Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol 472:595–614
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic, New York
Medbo JI, Tabata I (1993) Anaerobic energy release in working muscle during 30s to 3 min of exhausting bicycling. J Appl Physiol 75:1654–1660
Oakley BR, Kirsch DR, Morris NR (1980) A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Analytical Biochemistry 105(1):361–363
Parolin ML, Chesley A, Matsos MP, Spriet LL, Jones NL, Heigenhauser GJF (1999) Regulation of skeletal muscle glycogen phoshorylase and PDH during maximal intermittent exercise. Am J Physiol 277:E890–E900
Rall JA, Woledge RC (1990) Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol 259:197–203
Ranatunga KW (1984) The force–velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol 351:517–529
Saltin B, Gagge AP, Stolwijk JAJ (1968) Muscle temperature during submaximal exercise in man. J Appl Physiol 25:679–688
Sargeant AJ, Jones DA (1995) The significance of motor unit variability in sustaining mechanical output of muscle. In: Gandevia SC, Enoka RM, McComas AJ, Stuart DG, Thomas CK (eds) Fatigue: neural and muscular mechanisms. Plenum, New York, pp 323–338
Sargeant AJ, Rademaker A (1996) Human muscle power in the locomotory range of contraction velocities increases with temperature due to an increase in power generated by type I fibres. J Physiol 491:128P
Spriet LL (1995) Anaerobic metabolism during high-intensity exercise. In: Hargreaves M (ed) Exercise metabolism. Human Kinetics, Illinois, pp 1–40
Spriet LL, Söderlund K, Bergstrom M, Hultman E (1987) Anaerobic energy release in skeletal muscle during electrical stimulation in men. J Appl Physiol 62:611–615
Starkie RL, Hargreaves M, Lambert DL, Proietto J, Febbraio MA (1999) Effect of temperature on muscle metabolism during submaximal exercise in humans. Exp Physiol 84:775–784
Steinen GJM, Kiers JL, Bottinelli R, Reggiani C (1996) Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre types and temperature dependence. J Physiol 493:299–307
Wibom R, Söderlund K, Lundin A, Hultman E (1991) A luminometric method for the determination of ATP and phosphocreatine in single human skeletal muscle fibres. J Biolumin and Chemilumin 6:123–129
Wyss M, Schlegel J, James P, Eppenberger HM, Wallimann T (1990) Mitochondrial creatine kinase from chicken brain. Purification, biophysical characterization, and generation of heterodimeric and heterooctameric molecules with subunits of other creatine kinase isoenzymes. J Biol Chem 265:15900–15908
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gray, S.R., Soderlund, K., Watson, M. et al. Skeletal muscle ATP turnover and single fibre ATP and PCr content during intense exercise at different muscle temperatures in humans. Pflugers Arch - Eur J Physiol 462, 885–893 (2011). https://doi.org/10.1007/s00424-011-1032-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-1032-4